 Found 374 hits with Last Name = 'ajani' and Initial = 'h'
Found 374 hits with Last Name = 'ajani' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Serine racemase
(Mus musculus) | BDBM50038343
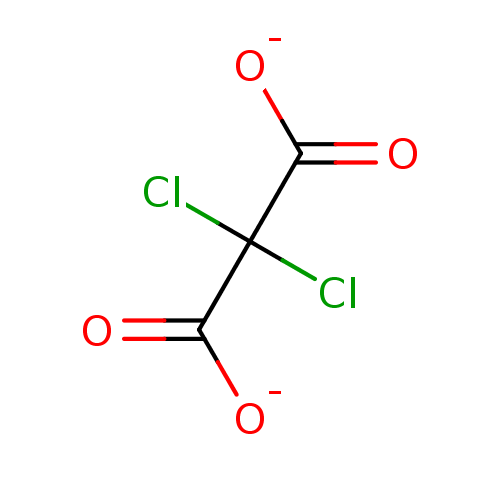
(CHEMBL3360546)Show InChI InChI=1S/C3H2Cl2O4.2Na/c4-3(5,1(6)7)2(8)9;;/h(H,6,7)(H,8,9);;/q;2*+1/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of mouse serine racemase by Lineweaver-Burk plot |
Eur J Med Chem 89: 189-97 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.043
BindingDB Entry DOI: 10.7270/Q22N53WK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Serine racemase
(Mus musculus) | BDBM14673
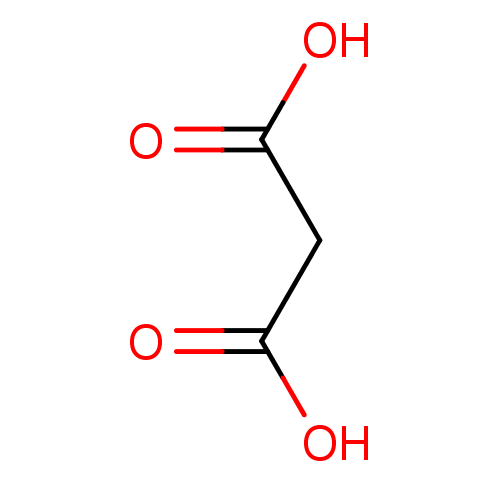
(Fragment 3 | Malonic Acid | propanedioic acid)Show InChI InChI=1S/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of mouse serine racemase by Lineweaver-Burk plot |
Eur J Med Chem 89: 189-97 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.043
BindingDB Entry DOI: 10.7270/Q22N53WK |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453198
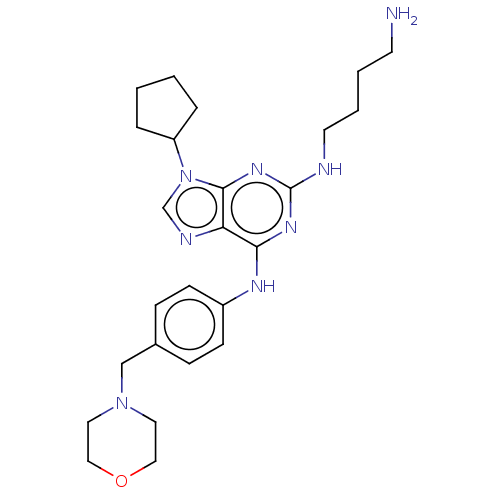
(CHEMBL4212560)Show SMILES NCCCCNc1nc(Nc2ccc(CN3CCOCC3)cc2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C25H36N8O/c26-11-3-4-12-27-25-30-23(22-24(31-25)33(18-28-22)21-5-1-2-6-21)29-20-9-7-19(8-10-20)17-32-13-15-34-16-14-32/h7-10,18,21H,1-6,11-17,26H2,(H2,27,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453202
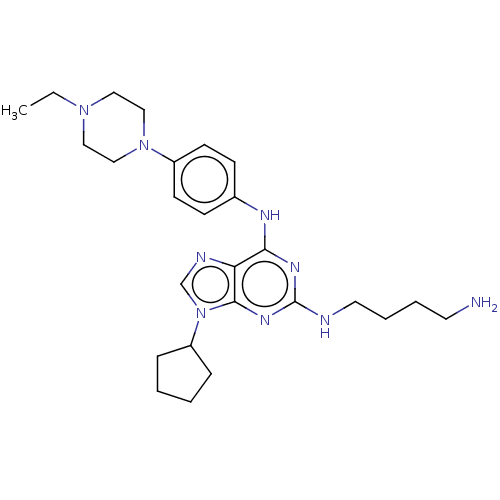
(CHEMBL4207317)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nc(NCCCCN)nc3n(cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H39N9/c1-2-33-15-17-34(18-16-33)21-11-9-20(10-12-21)30-24-23-25(32-26(31-24)28-14-6-5-13-27)35(19-29-23)22-7-3-4-8-22/h9-12,19,22H,2-8,13-18,27H2,1H3,(H2,28,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16314
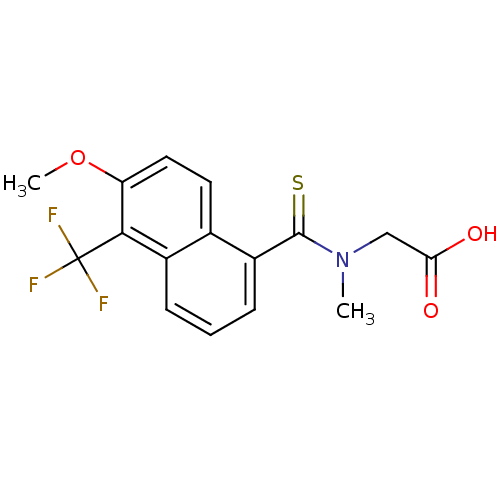
(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire
| Assay Description
The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... |
ACS Chem Biol 11: 2693-2705 (2016)
Article DOI: 10.1021/acschembio.6b00382
BindingDB Entry DOI: 10.7270/Q2NG4PFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50576576
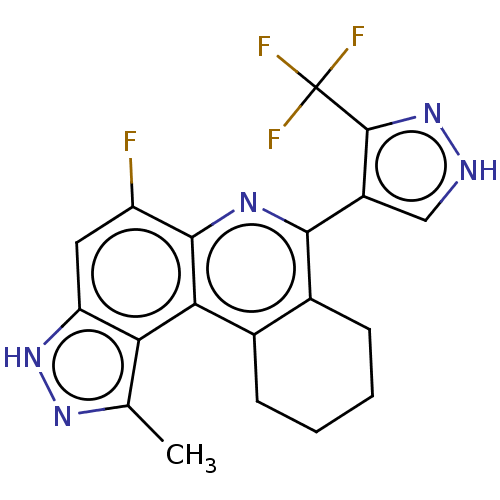
(CHEMBL4869569)Show SMILES Cc1n[nH]c2cc(F)c3nc(-c4c[nH]nc4C(F)(F)F)c4CCCCc4c3c12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM16314

(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire
| Assay Description
The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... |
ACS Chem Biol 11: 2693-2705 (2016)
Article DOI: 10.1021/acschembio.6b00382
BindingDB Entry DOI: 10.7270/Q2NG4PFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
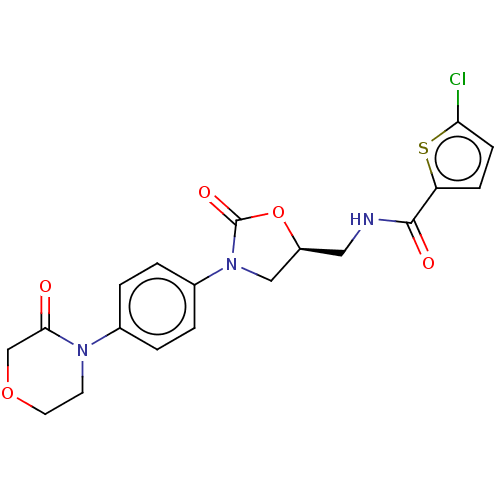
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453185

(CHEMBL4203825)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nc(N[C@H]3CC[C@H](N)CC3)nc3n(cnc23)C2CCCC2)cc1 |r,wU:17.17,wD:20.21,(51.74,-32.62,;50.97,-31.28,;49.43,-31.28,;48.66,-29.95,;47.12,-29.95,;46.35,-31.28,;47.12,-32.62,;48.66,-32.62,;44.81,-31.28,;44.04,-32.62,;42.5,-32.62,;41.73,-31.28,;40.19,-31.28,;39.42,-29.95,;40.19,-28.62,;39.42,-27.29,;40.19,-25.95,;41.73,-25.95,;42.5,-24.62,;44.04,-24.62,;44.81,-25.95,;46.35,-25.95,;44.04,-27.29,;42.5,-27.29,;37.88,-27.29,;37.11,-28.62,;35.6,-28.94,;35.44,-30.47,;36.85,-31.09,;37.88,-29.95,;34.46,-27.9,;32.95,-28.23,;32.18,-26.89,;33.22,-25.75,;34.62,-26.37,;42.5,-29.95,;44.04,-29.95,)| Show InChI InChI=1S/C28H41N9/c1-2-35-15-17-36(18-16-35)23-13-11-21(12-14-23)31-26-25-27(37(19-30-25)24-5-3-4-6-24)34-28(33-26)32-22-9-7-20(29)8-10-22/h11-14,19-20,22,24H,2-10,15-18,29H2,1H3,(H2,31,32,33,34)/t20-,22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) D835Y mutant expressed in Sf9 insect cells using poly (Ala,Glu... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453197

(CHEMBL4208701)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(cc2)N2CCOCC2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(26.32,-12.53,;24.78,-12.53,;24.01,-11.19,;22.47,-11.19,;21.7,-12.53,;22.47,-13.86,;24.01,-13.86,;20.16,-12.53,;19.39,-13.86,;20.16,-15.19,;19.39,-16.53,;20.16,-17.86,;21.7,-17.86,;22.47,-16.53,;24.01,-16.53,;24.78,-17.86,;24.01,-19.19,;22.47,-19.19,;26.32,-17.86,;27.09,-16.53,;28.63,-16.53,;29.4,-17.86,;28.63,-19.19,;27.09,-19.19,;17.85,-16.53,;16.82,-17.67,;15.41,-17.05,;15.58,-15.51,;14.43,-14.48,;12.93,-14.8,;12.16,-13.47,;13.19,-12.33,;14.59,-12.95,;17.08,-15.19,;17.85,-13.86,)| Show InChI InChI=1S/C26H36N8O/c27-18-5-7-20(8-6-18)30-26-31-24(23-25(32-26)34(17-28-23)22-3-1-2-4-22)29-19-9-11-21(12-10-19)33-13-15-35-16-14-33/h9-12,17-18,20,22H,1-8,13-16,27H2,(H2,29,30,31,32)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392595
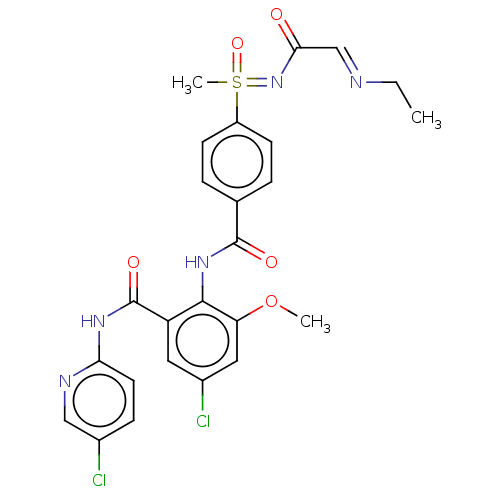
(CHEMBL2153392)Show SMILES CC\N=C\C(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H25Cl2N5O5S/c1-4-28-14-22(33)32-38(3,36)18-8-5-15(6-9-18)24(34)31-23-19(11-17(27)12-20(23)37-2)25(35)30-21-10-7-16(26)13-29-21/h5-14,38H,4H2,1-3H3,(H,31,34)(H,29,30,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16313
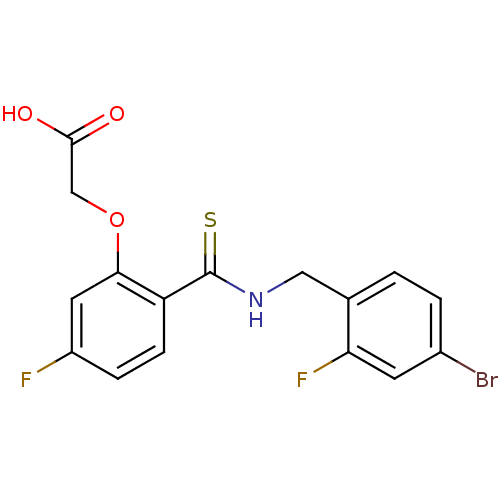
(2-(2-{[(4-bromo-2-fluorophenyl)methyl]carbamothioy...)Show InChI InChI=1S/C16H12BrF2NO3S/c17-10-2-1-9(13(19)5-10)7-20-16(24)12-4-3-11(18)6-14(12)23-8-15(21)22/h1-6H,7-8H2,(H,20,24)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire
| Assay Description
The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... |
ACS Chem Biol 11: 2693-2705 (2016)
Article DOI: 10.1021/acschembio.6b00382
BindingDB Entry DOI: 10.7270/Q2NG4PFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453202

(CHEMBL4207317)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nc(NCCCCN)nc3n(cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H39N9/c1-2-33-15-17-34(18-16-33)21-11-9-20(10-12-21)30-24-23-25(32-26(31-24)28-14-6-5-13-27)35(19-29-23)22-7-3-4-8-22/h9-12,19,22H,2-8,13-18,27H2,1H3,(H2,28,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) D835Y mutant expressed in Sf9 insect cells using poly (Ala,Glu... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453185

(CHEMBL4203825)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nc(N[C@H]3CC[C@H](N)CC3)nc3n(cnc23)C2CCCC2)cc1 |r,wU:17.17,wD:20.21,(51.74,-32.62,;50.97,-31.28,;49.43,-31.28,;48.66,-29.95,;47.12,-29.95,;46.35,-31.28,;47.12,-32.62,;48.66,-32.62,;44.81,-31.28,;44.04,-32.62,;42.5,-32.62,;41.73,-31.28,;40.19,-31.28,;39.42,-29.95,;40.19,-28.62,;39.42,-27.29,;40.19,-25.95,;41.73,-25.95,;42.5,-24.62,;44.04,-24.62,;44.81,-25.95,;46.35,-25.95,;44.04,-27.29,;42.5,-27.29,;37.88,-27.29,;37.11,-28.62,;35.6,-28.94,;35.44,-30.47,;36.85,-31.09,;37.88,-29.95,;34.46,-27.9,;32.95,-28.23,;32.18,-26.89,;33.22,-25.75,;34.62,-26.37,;42.5,-29.95,;44.04,-29.95,)| Show InChI InChI=1S/C28H41N9/c1-2-35-15-17-36(18-16-35)23-13-11-21(12-14-23)31-26-25-27(37(19-30-25)24-5-3-4-6-24)34-28(33-26)32-22-9-7-20(29)8-10-22/h11-14,19-20,22,24H,2-10,15-18,29H2,1H3,(H2,31,32,33,34)/t20-,22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453194

(CHEMBL4204373)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(CN3CCOCC3)cc2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(23.34,-10.58,;22.01,-11.35,;20.68,-10.58,;19.34,-11.35,;19.34,-12.89,;20.68,-13.66,;22.01,-12.89,;18.01,-13.66,;18.01,-15.2,;19.34,-15.97,;19.34,-17.51,;20.68,-18.28,;22.01,-17.51,;22.01,-15.97,;23.34,-15.2,;24.68,-15.97,;26.01,-15.2,;27.34,-15.97,;28.68,-15.2,;30.01,-15.97,;30.01,-17.51,;28.68,-18.28,;27.34,-17.51,;24.68,-17.51,;23.34,-18.28,;18.01,-18.28,;17.69,-19.79,;16.16,-19.95,;15.53,-18.54,;14.02,-18.22,;12.88,-19.25,;11.55,-18.48,;11.87,-16.98,;13.4,-16.82,;16.67,-17.51,;16.67,-15.97,)| Show InChI InChI=1S/C27H38N8O/c28-20-7-11-22(12-8-20)31-27-32-25(24-26(33-27)35(18-29-24)23-3-1-2-4-23)30-21-9-5-19(6-10-21)17-34-13-15-36-16-14-34/h5-6,9-10,18,20,22-23H,1-4,7-8,11-17,28H2,(H2,30,31,32,33)/t20-,22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453200

(CHEMBL4210745)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(cc2)N2CCCC2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(26.86,-12.53,;25.32,-12.53,;24.55,-11.19,;23.01,-11.19,;22.24,-12.53,;23.01,-13.86,;24.55,-13.86,;20.7,-12.53,;19.93,-13.86,;20.7,-15.19,;19.93,-16.53,;20.7,-17.86,;22.24,-17.86,;23.01,-16.53,;24.55,-16.53,;25.32,-17.86,;24.55,-19.19,;23.01,-19.19,;26.86,-17.86,;27.76,-16.62,;29.23,-17.09,;29.23,-18.63,;27.76,-19.11,;18.39,-16.53,;17.36,-17.67,;15.95,-17.05,;16.11,-15.51,;14.97,-14.48,;13.46,-14.8,;12.69,-13.47,;13.72,-12.33,;15.13,-12.95,;17.62,-15.19,;18.39,-13.86,)| Show InChI InChI=1S/C26H36N8/c27-18-7-9-20(10-8-18)30-26-31-24(23-25(32-26)34(17-28-23)22-5-1-2-6-22)29-19-11-13-21(14-12-19)33-15-3-4-16-33/h11-14,17-18,20,22H,1-10,15-16,27H2,(H2,29,30,31,32)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50576570

(CHEMBL4871182)Show SMILES OCc1n[nH]c2ccc3nc(-c4c[nH]nc4C(F)(F)F)c4CCCCc4c3c12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50576569

(CHEMBL4853855)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]nc(Br)c3c2c2CCCCc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50576545

(CHEMBL4866768)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]ncc3c2c2CCCCCc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50139171
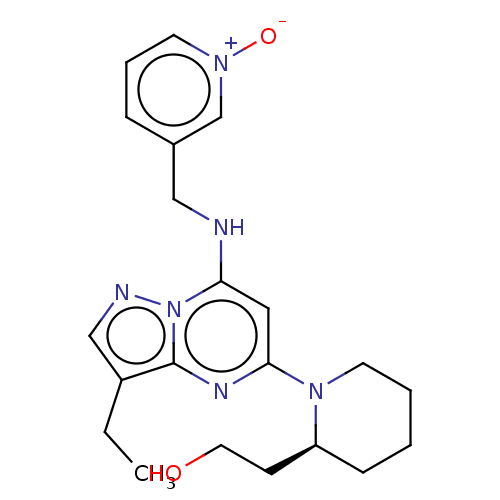
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK2/Cyclin A1 (unknown origin) at 1 uM using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392593
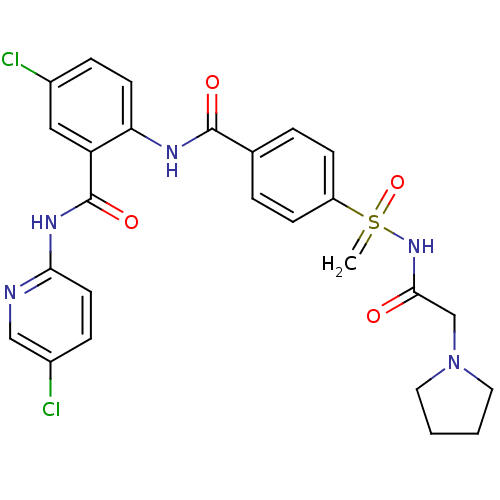
(CHEMBL2153383)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)S(=C)(=O)NC(=O)CN2CCCC2)nc1 Show InChI InChI=1S/C26H25Cl2N5O4S/c1-38(37,32-24(34)16-33-12-2-3-13-33)20-8-4-17(5-9-20)25(35)30-22-10-6-18(27)14-21(22)26(36)31-23-11-7-19(28)15-29-23/h4-11,14-15H,1-3,12-13,16H2,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392596
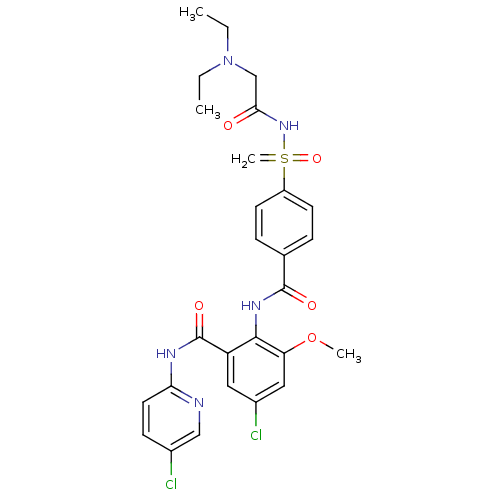
(CHEMBL2153393)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H29Cl2N5O5S/c1-5-34(6-2)16-24(35)33-40(4,38)20-10-7-17(8-11-20)26(36)32-25-21(13-19(29)14-22(25)39-3)27(37)31-23-12-9-18(28)15-30-23/h7-15H,4-6,16H2,1-3H3,(H,32,36)(H,30,31,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453234

(CHEMBL4213717)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(nc2)N2CCOCC2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(26.32,-12.34,;24.78,-12.34,;24.01,-11,;22.47,-11,;21.7,-12.34,;22.47,-13.67,;24.01,-13.67,;20.16,-12.34,;19.39,-13.67,;20.16,-15,;19.39,-16.34,;20.16,-17.67,;21.7,-17.67,;22.47,-16.34,;24.01,-16.34,;24.78,-17.67,;24.01,-19,;22.47,-19,;26.32,-17.67,;27.09,-16.34,;28.63,-16.34,;29.4,-17.67,;28.63,-19,;27.09,-19,;17.85,-16.34,;16.82,-17.48,;15.41,-16.85,;15.58,-15.32,;14.43,-14.29,;12.93,-14.61,;12.16,-13.28,;13.19,-12.13,;14.59,-12.76,;17.08,-15,;17.85,-13.67,)| Show InChI InChI=1S/C25H35N9O/c26-17-5-7-18(8-6-17)30-25-31-23(22-24(32-25)34(16-28-22)20-3-1-2-4-20)29-19-9-10-21(27-15-19)33-11-13-35-14-12-33/h9-10,15-18,20H,1-8,11-14,26H2,(H2,29,30,31,32)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453196

(CHEMBL4216401)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(Br)cc2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(13.6,-16.81,;15.14,-16.81,;15.91,-18.14,;17.45,-18.14,;18.22,-16.81,;17.45,-15.47,;15.91,-15.47,;19.76,-16.81,;20.53,-15.47,;19.76,-14.14,;20.53,-12.8,;19.76,-11.47,;18.22,-11.47,;17.45,-12.8,;15.91,-12.8,;15.14,-11.47,;13.6,-11.47,;15.91,-10.14,;17.45,-10.14,;22.07,-12.8,;23.1,-11.66,;24.51,-12.29,;24.35,-13.82,;25.49,-14.85,;27,-14.53,;27.77,-15.86,;26.74,-17.01,;25.33,-16.38,;22.84,-14.14,;22.07,-15.47,)| Show InChI InChI=1S/C22H28BrN7/c23-14-5-9-16(10-6-14)26-20-19-21(30(13-25-19)18-3-1-2-4-18)29-22(28-20)27-17-11-7-15(24)8-12-17/h5-6,9-10,13,15,17-18H,1-4,7-8,11-12,24H2,(H2,26,27,28,29)/t15-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) D835Y mutant expressed in Sf9 insect cells using poly (Ala,Glu... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392590
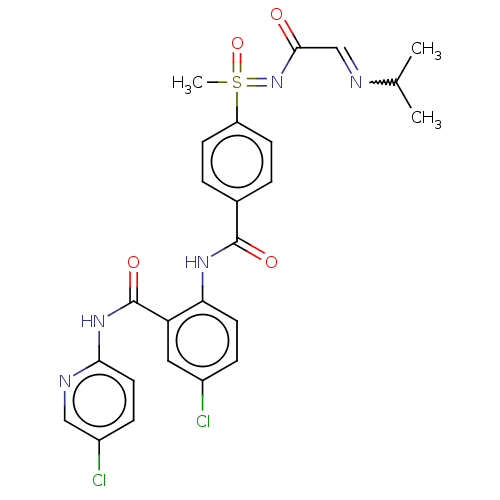
(CHEMBL2153378)Show SMILES CC(C)N=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O4S/c1-15(2)28-14-23(33)32-37(3,36)19-8-4-16(5-9-19)24(34)30-21-10-6-17(26)12-20(21)25(35)31-22-11-7-18(27)13-29-22/h4-15,37H,1-3H3,(H,30,34)(H,29,31,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453196

(CHEMBL4216401)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(Br)cc2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(13.6,-16.81,;15.14,-16.81,;15.91,-18.14,;17.45,-18.14,;18.22,-16.81,;17.45,-15.47,;15.91,-15.47,;19.76,-16.81,;20.53,-15.47,;19.76,-14.14,;20.53,-12.8,;19.76,-11.47,;18.22,-11.47,;17.45,-12.8,;15.91,-12.8,;15.14,-11.47,;13.6,-11.47,;15.91,-10.14,;17.45,-10.14,;22.07,-12.8,;23.1,-11.66,;24.51,-12.29,;24.35,-13.82,;25.49,-14.85,;27,-14.53,;27.77,-15.86,;26.74,-17.01,;25.33,-16.38,;22.84,-14.14,;22.07,-15.47,)| Show InChI InChI=1S/C22H28BrN7/c23-14-5-9-16(10-6-14)26-20-19-21(30(13-25-19)18-3-1-2-4-18)29-22(28-20)27-17-11-7-15(24)8-12-17/h5-6,9-10,13,15,17-18H,1-4,7-8,11-12,24H2,(H2,26,27,28,29)/t15-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453195

(CHEMBL4202430)Show SMILES NCCCCNc1nc(Nc2ccc(cc2)N2CCOCC2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C24H34N8O/c25-11-3-4-12-26-24-29-22(21-23(30-24)32(17-27-21)20-5-1-2-6-20)28-18-7-9-19(10-8-18)31-13-15-33-16-14-31/h7-10,17,20H,1-6,11-16,25H2,(H2,26,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453192

(CHEMBL4216043)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(cc2)N2CC3(COC3)C2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(16.44,-9.37,;17.21,-10.71,;16.44,-12.04,;17.21,-13.38,;18.75,-13.38,;19.52,-12.04,;18.75,-10.71,;19.52,-14.71,;18.75,-16.04,;19.52,-17.38,;18.75,-18.71,;19.52,-20.04,;21.06,-20.04,;21.83,-18.71,;23.37,-18.71,;24.14,-20.04,;23.37,-21.38,;21.83,-21.38,;25.68,-20.04,;26.77,-21.13,;27.86,-20.04,;28.95,-18.96,;30.04,-20.04,;28.95,-21.13,;26.77,-18.96,;17.21,-18.71,;16.18,-19.85,;14.78,-19.23,;14.94,-17.7,;13.79,-16.67,;12.29,-16.99,;11.52,-15.65,;12.55,-14.51,;13.95,-15.13,;16.44,-17.38,;17.21,-16.04,)| Show InChI InChI=1S/C27H36N8O/c28-18-5-7-20(8-6-18)31-26-32-24(23-25(33-26)35(17-29-23)22-3-1-2-4-22)30-19-9-11-21(12-10-19)34-13-27(14-34)15-36-16-27/h9-12,17-18,20,22H,1-8,13-16,28H2,(H2,30,31,32,33)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50576552

(CHEMBL4869214)Show SMILES CCc1c(C)c(nc2ccc3[nH]ncc3c12)-c1c[nH]nc1C(F)(F)F | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453235

(CHEMBL4207535)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(Cl)cc2)c2ncn(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(7.16,-31.99,;8.7,-31.99,;9.47,-33.32,;11.01,-33.32,;11.78,-31.99,;11.01,-30.66,;9.47,-30.66,;13.32,-31.99,;14.09,-30.66,;13.32,-29.32,;14.09,-27.99,;13.32,-26.65,;11.78,-26.65,;11.01,-27.99,;9.47,-27.99,;8.7,-26.65,;7.16,-26.65,;9.47,-25.32,;11.01,-25.32,;15.63,-27.99,;16.66,-26.84,;18.07,-27.47,;17.91,-29,;19.05,-30.03,;20.56,-29.71,;21.33,-31.05,;20.3,-32.19,;18.89,-31.56,;16.4,-29.32,;15.63,-30.66,)| Show InChI InChI=1S/C22H28ClN7/c23-14-5-9-16(10-6-14)26-20-19-21(30(13-25-19)18-3-1-2-4-18)29-22(28-20)27-17-11-7-15(24)8-12-17/h5-6,9-10,13,15,17-18H,1-4,7-8,11-12,24H2,(H2,26,27,28,29)/t15-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453198

(CHEMBL4212560)Show SMILES NCCCCNc1nc(Nc2ccc(CN3CCOCC3)cc2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C25H36N8O/c26-11-3-4-12-27-25-30-23(22-24(31-25)33(18-28-22)21-5-1-2-6-21)29-20-9-7-19(8-10-20)17-32-13-15-34-16-14-32/h7-10,18,21H,1-6,11-17,26H2,(H2,27,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) D835Y mutant expressed in Sf9 insect cells using poly (Ala,Glu... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50510914
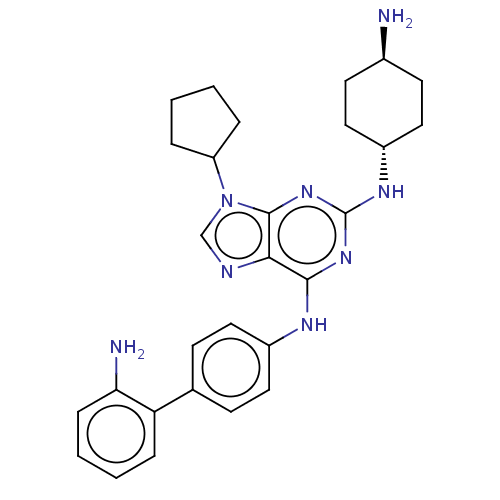
(CHEMBL4558664)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(cc2)-c2ccccc2N)c2ncn(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(50.31,-9.1,;51.64,-9.88,;52.97,-9.12,;54.3,-9.89,;54.3,-11.44,;52.96,-12.2,;51.63,-11.42,;55.63,-12.21,;56.97,-11.44,;56.97,-9.89,;58.3,-9.12,;58.29,-7.58,;59.62,-6.81,;60.96,-7.58,;62.29,-6.81,;62.28,-5.26,;60.94,-4.5,;59.61,-5.28,;63.61,-4.49,;63.6,-2.96,;64.93,-2.18,;66.27,-2.94,;66.28,-4.48,;64.95,-5.26,;64.95,-6.8,;59.63,-9.89,;61.1,-9.41,;62.01,-10.66,;61.1,-11.91,;61.58,-13.37,;60.67,-14.61,;61.58,-15.86,;63.04,-15.38,;63.04,-13.84,;59.63,-11.43,;58.3,-12.21,)| Show InChI InChI=1S/C28H34N8/c29-19-11-15-21(16-12-19)33-28-34-26(25-27(35-28)36(17-31-25)22-5-1-2-6-22)32-20-13-9-18(10-14-20)23-7-3-4-8-24(23)30/h3-4,7-10,13-14,17,19,21-22H,1-2,5-6,11-12,15-16,29-30H2,(H2,32,33,34,35)/t19-,21- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University and Institute of Experimental Botany
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 3C cleavage site/N-terminal GST-His6 fused PDGFR alpha C-terminal fragment (Q551 to L1089 residues) harboring V561D m... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111663
BindingDB Entry DOI: 10.7270/Q2M90D08 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50515042
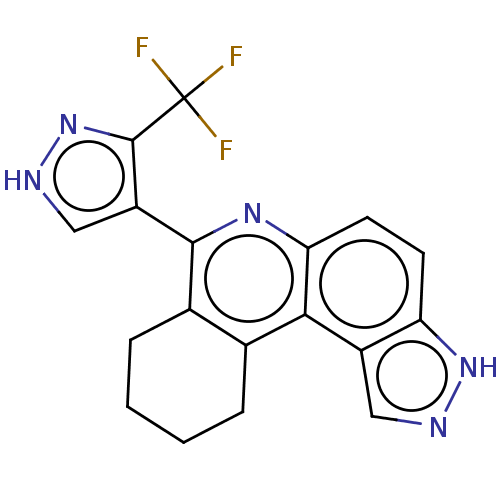
(CHEMBL4593398)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]ncc3c2c2CCCCc12 Show InChI InChI=1S/C18H14F3N5/c19-18(20,21)17-12(8-23-26-17)16-10-4-2-1-3-9(10)15-11-7-22-25-13(11)5-6-14(15)24-16/h5-8H,1-4H2,(H,22,25)(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00330
BindingDB Entry DOI: 10.7270/Q2862M9D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453193

(CHEMBL4213103)Show SMILES NCCCCCNc1nc(Nc2ccc(CN3CCOCC3)cc2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C26H38N8O/c27-12-4-1-5-13-28-26-31-24(23-25(32-26)34(19-29-23)22-6-2-3-7-22)30-21-10-8-20(9-11-21)18-33-14-16-35-17-15-33/h8-11,19,22H,1-7,12-18,27H2,(H2,28,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant human FLT3 (R571 to S993 residues) ITD mutant expressed in Sf9 insect cells using poly (Ala,Glu,L... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392588
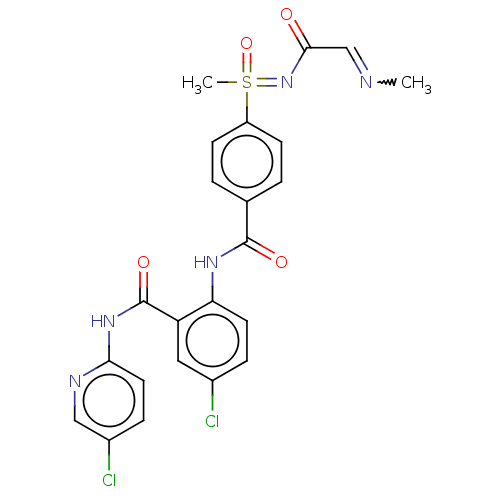
(CHEMBL2153376)Show SMILES CN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:1.0| Show InChI InChI=1S/C23H21Cl2N5O4S/c1-26-13-21(31)30-35(2,34)17-7-3-14(4-8-17)22(32)28-19-9-5-15(24)11-18(19)23(33)29-20-10-6-16(25)12-27-20/h3-13,35H,1-2H3,(H,28,32)(H,27,29,33)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392594
(CHEMBL2153391)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)S(C)(=O)=NC(=O)C=NC |w:35.38| Show InChI InChI=1S/C24H23Cl2N5O5S/c1-27-13-21(32)31-37(3,35)17-7-4-14(5-8-17)23(33)30-22-18(10-16(26)11-19(22)36-2)24(34)29-20-9-6-15(25)12-28-20/h4-13,37H,1-3H3,(H,30,33)(H,28,29,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50453198

(CHEMBL4212560)Show SMILES NCCCCNc1nc(Nc2ccc(CN3CCOCC3)cc2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C25H36N8O/c26-11-3-4-12-27-25-30-23(22-24(31-25)33(18-28-22)21-5-1-2-6-21)29-20-9-7-19(8-10-20)17-32-13-15-34-16-14-32/h7-10,18,21H,1-6,11-17,26H2,(H2,27,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST/His6-fused recombinant wild type human FLT3 (R571 to S993 residues) expressed in Sf9 insect cells using poly (Ala,Glu,Ly... |
J Med Chem 61: 3855-3869 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01529
BindingDB Entry DOI: 10.7270/Q2JH3PR1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50510932
(CHEMBL4576574)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ccc(cc2)-n2cnnc2)c2ncn(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(18.68,-24.67,;20.01,-25.45,;21.35,-24.69,;22.68,-25.46,;22.68,-27.01,;21.34,-27.77,;20.01,-26.99,;24.01,-27.78,;25.34,-27.01,;25.34,-25.46,;26.67,-24.69,;26.67,-23.15,;28,-22.38,;29.33,-23.15,;30.66,-22.38,;30.66,-20.84,;29.31,-20.07,;27.99,-20.85,;31.98,-20.06,;33.39,-20.68,;34.42,-19.53,;33.64,-18.2,;32.14,-18.52,;28.01,-25.46,;29.48,-24.98,;30.38,-26.23,;29.48,-27.48,;29.95,-28.94,;29.05,-30.19,;29.95,-31.43,;31.42,-30.96,;31.42,-29.42,;28.01,-27,;26.68,-27.78,)| Show InChI InChI=1S/C24H30N10/c25-16-5-7-18(8-6-16)30-24-31-22(21-23(32-24)34(13-26-21)20-3-1-2-4-20)29-17-9-11-19(12-10-17)33-14-27-28-15-33/h9-16,18,20H,1-8,25H2,(H2,29,30,31,32)/t16-,18- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University and Institute of Experimental Botany
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 3C cleavage site/N-terminal GST-His6 fused PDGFR alpha C-terminal fragment (Q551 to L1089 residues) harboring V561D m... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111663
BindingDB Entry DOI: 10.7270/Q2M90D08 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data