 Found 251 hits with Last Name = 'akhtar' and Initial = 'm'
Found 251 hits with Last Name = 'akhtar' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591808

(CHEMBL5207397)Show SMILES OC(=O)[C@@H]1CCCN(CC\C=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591807

(CHEMBL5178760)Show SMILES OC(=O)C1CCCN(CC\C=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591806

(CHEMBL5205864)Show SMILES Cl.OC(=O)C1CCCN(CCO\N=C(/Cc2ccc(F)cc2)c2ccc(F)cc2)C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50039251

((R)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)pi...)Show SMILES [#6]-c1ccsc1\[#6](=[#6]/[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6@H](-[#6]-1)-[#6](-[#8])=O)-c1sccc1-[#6] |r| Show InChI InChI=1S/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50591809
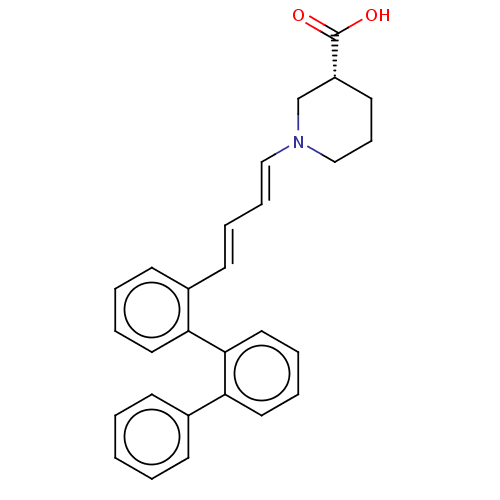
(CHEMBL5203365)Show SMILES OC(=O)[C@@H]1CCCN(C1)\C=C\C=C\c1ccccc1-c1ccccc1-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114269
BindingDB Entry DOI: 10.7270/Q2JM2FM0 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50445253

(CHEMBL1232500)Show InChI InChI=1S/C12H13NO2S/c1-3-13-10-7-9(15)4-5-11(10)16-12(13)6-8(2)14/h4-7,15H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50041419
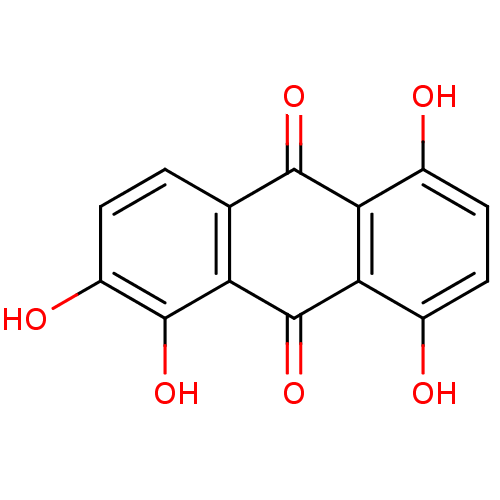
(1,2,5,8-tetrahydroxy-9,10-anthracenedione | 1,2,5,...)Show InChI InChI=1S/C14H8O6/c15-6-3-4-7(16)11-10(6)12(18)5-1-2-8(17)13(19)9(5)14(11)20/h1-4,15-17,19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403
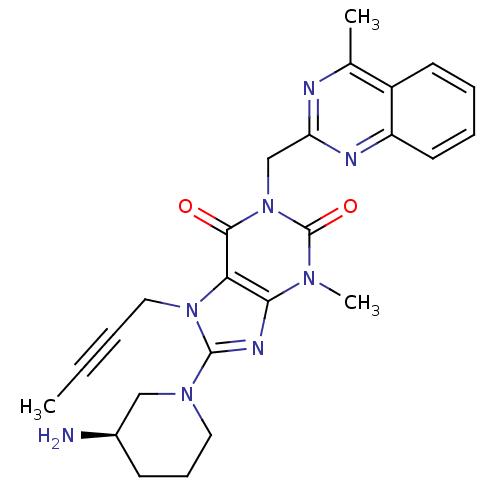
((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50065646

(CHEMBL3087181)Show SMILES O=C(N1CCC(CC1)C(c1ccc2OCOc2c1)c1ccc2OCOc2c1)n1cncn1 Show InChI InChI=1S/C23H22N4O5/c28-23(27-12-24-11-25-27)26-7-5-15(6-8-26)22(16-1-3-18-20(9-16)31-13-29-18)17-2-4-19-21(10-17)32-14-30-19/h1-4,9-12,15,22H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222244
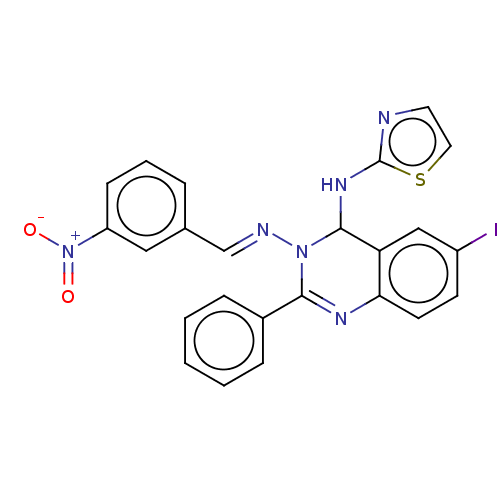
(6-Iodo-N3-(3-nitrobenzylidene)-2-phenyl-N4-(thiazo...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)c1 |c:27| Show InChI InChI=1S/C24H17IN6O2S/c25-18-9-10-21-20(14-18)23(29-24-26-11-12-34-24)30(22(28-21)17-6-2-1-3-7-17)27-15-16-5-4-8-19(13-16)31(32)33/h1-15,23H,(H,26,29)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50242634

(5-((biphenyl-4-yl)methyl)-N,N-dimethyl-2H-tetrazol...)Show InChI InChI=1S/C17H17N5O/c1-21(2)17(23)22-19-16(18-20-22)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222254

(6-Iodo-N4-(4-methylthiazol-2-yl)-N3-(3-nitrobenzyl...)Show SMILES Cc1csc(NC2N(\N=C\c3cccc(c3)[N+]([O-])=O)C(=Nc3ccc(I)cc23)c2ccccc2)n1 |c:20| Show InChI InChI=1S/C25H19IN6O2S/c1-16-15-35-25(28-16)30-24-21-13-19(26)10-11-22(21)29-23(18-7-3-2-4-8-18)31(24)27-14-17-6-5-9-20(12-17)32(33)34/h2-15,24H,1H3,(H,28,30)/b27-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222239
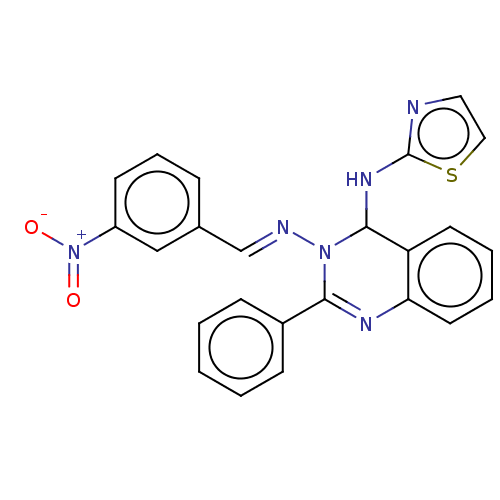
(N3-(3-Nitrobenzylidene)-2-phenyl-N4-(thiazol-2-yl)...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)c1 |c:26| Show InChI InChI=1S/C24H18N6O2S/c31-30(32)19-10-6-7-17(15-19)16-26-29-22(18-8-2-1-3-9-18)27-21-12-5-4-11-20(21)23(29)28-24-25-13-14-33-24/h1-16,23H,(H,25,28)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222245
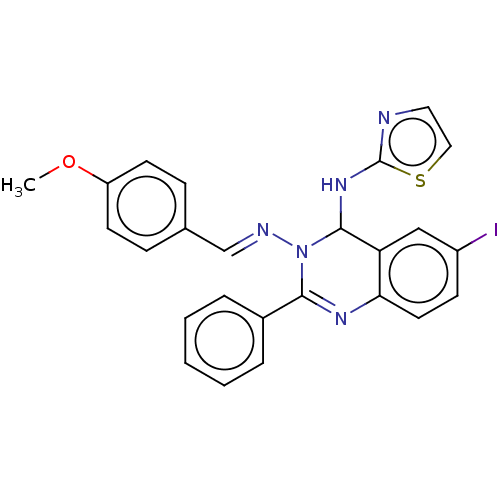
(6-Iodo-N3-(4-methoxybenzylidene)-2-phenyl-N4-(thia...)Show SMILES COc1ccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C25H20IN5OS/c1-32-20-10-7-17(8-11-20)16-28-31-23(18-5-3-2-4-6-18)29-22-12-9-19(26)15-21(22)24(31)30-25-27-13-14-33-25/h2-16,24H,1H3,(H,27,30)/b28-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222255
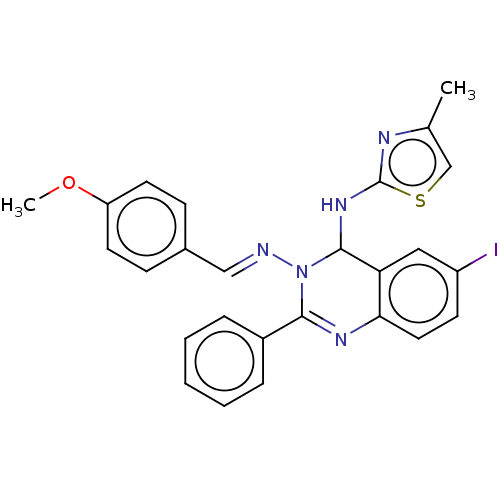
(6-Iodo-N3-(4-methoxybenzylidene)-N4-(4-methylthiaz...)Show SMILES COc1ccc(\C=N\N2C(Nc3nc(C)cs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C26H22IN5OS/c1-17-16-34-26(29-17)31-25-22-14-20(27)10-13-23(22)30-24(19-6-4-3-5-7-19)32(25)28-15-18-8-11-21(33-2)12-9-18/h3-16,25H,1-2H3,(H,29,31)/b28-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222249
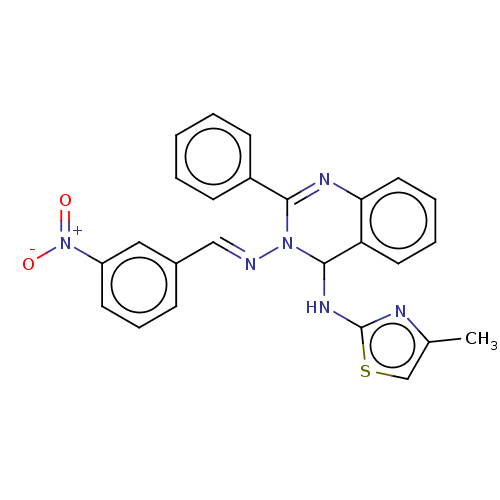
(N4-(4-Methylthiazol-2-yl)-N3-(3-nitrobenzylidene)-...)Show SMILES Cc1csc(NC2N(\N=C\c3cccc(c3)[N+]([O-])=O)C(=Nc3ccccc23)c2ccccc2)n1 |c:20| Show InChI InChI=1S/C25H20N6O2S/c1-17-16-34-25(27-17)29-24-21-12-5-6-13-22(21)28-23(19-9-3-2-4-10-19)30(24)26-15-18-8-7-11-20(14-18)31(32)33/h2-16,24H,1H3,(H,27,29)/b26-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222250

(N3-(4-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...)Show SMILES COc1ccc(\C=N\N2C(Nc3nc(C)cs3)c3ccccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C26H23N5OS/c1-18-17-33-26(28-18)30-25-22-10-6-7-11-23(22)29-24(20-8-4-3-5-9-20)31(25)27-16-19-12-14-21(32-2)15-13-19/h3-17,25H,1-2H3,(H,28,30)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222240
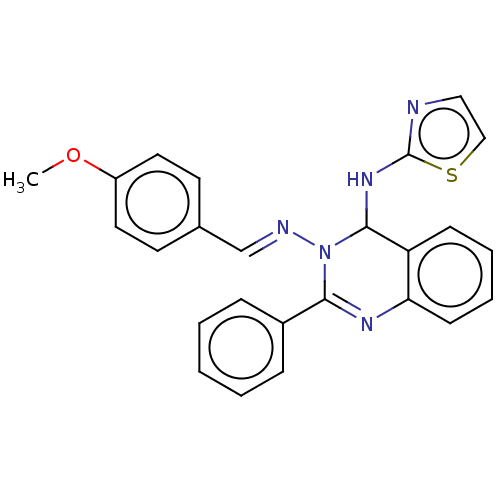
(N3-(4-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...)Show SMILES COc1ccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)cc1 |c:24| Show InChI InChI=1S/C25H21N5OS/c1-31-20-13-11-18(12-14-20)17-27-30-23(19-7-3-2-4-8-19)28-22-10-6-5-9-21(22)24(30)29-25-26-15-16-32-25/h2-17,24H,1H3,(H,26,29)/b27-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222247
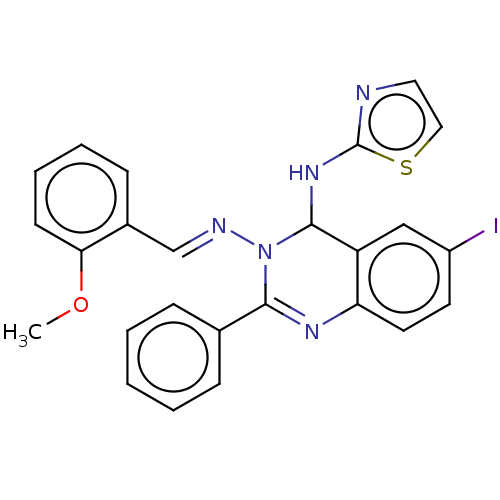
(6-Iodo-N3-(2-methoxybenzylidene)-2-phenyl-N4-(thia...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nccs2)c2cc(I)ccc2N=C1c1ccccc1 |c:28| Show InChI InChI=1S/C25H20IN5OS/c1-32-22-10-6-5-9-18(22)16-28-31-23(17-7-3-2-4-8-17)29-21-12-11-19(26)15-20(21)24(31)30-25-27-13-14-33-25/h2-16,24H,1H3,(H,27,30)/b28-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222257
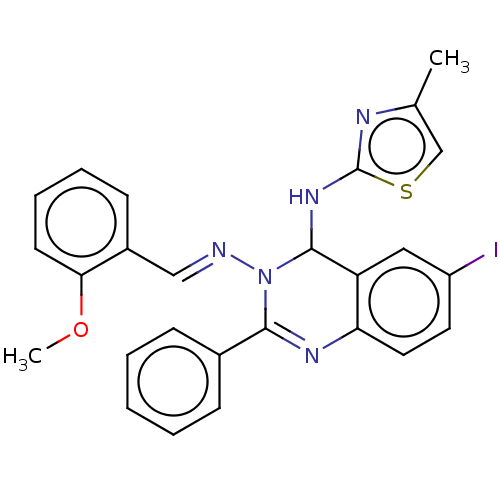
(6-Iodo-N3-(2-methoxybenzylidene)-N4-(4-methylthiaz...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nc(C)cs2)c2cc(I)ccc2N=C1c1ccccc1 |c:29| Show InChI InChI=1S/C26H22IN5OS/c1-17-16-34-26(29-17)31-25-21-14-20(27)12-13-22(21)30-24(18-8-4-3-5-9-18)32(25)28-15-19-10-6-7-11-23(19)33-2/h3-16,25H,1-2H3,(H,29,31)/b28-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222252
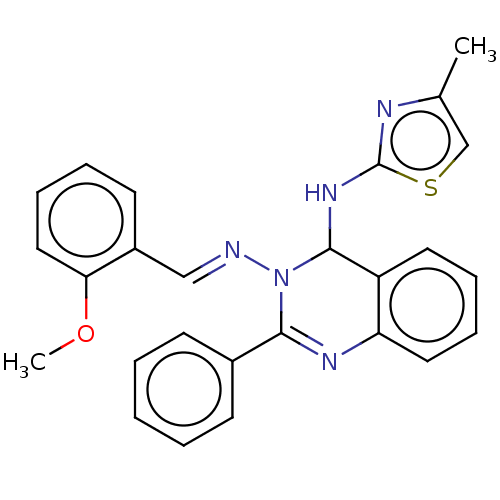
(N3-(2-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nc(C)cs2)c2ccccc2N=C1c1ccccc1 |c:28| Show InChI InChI=1S/C26H23N5OS/c1-18-17-33-26(28-18)30-25-21-13-7-8-14-22(21)29-24(19-10-4-3-5-11-19)31(25)27-16-20-12-6-9-15-23(20)32-2/h3-17,25H,1-2H3,(H,28,30)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM179929

(US9133148, 1a)Show SMILES OC(C1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H21F6NO7/c25-23(26,27)20(24(28,29)30)38-21(32)31-7-5-13(6-8-31)22(33,14-1-3-16-18(9-14)36-11-34-16)15-2-4-17-19(10-15)37-12-35-17/h1-4,9-10,13,20,33H,5-8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL transfected in human HEK293T cells assessed as reduction in ABPP binding by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM60622
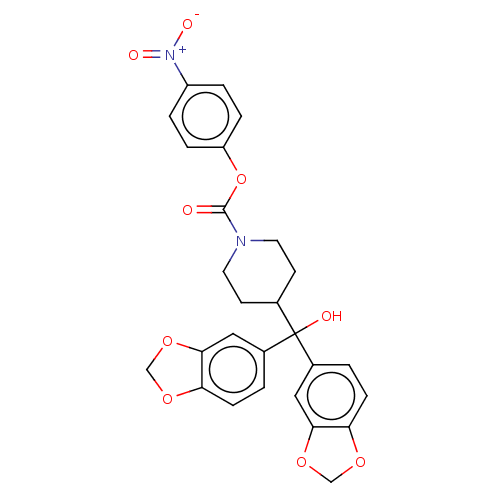
(BDBM50300355 | US11753371, Compound JZL-184 | US91...)Show SMILES OC(C1CCN(CC1)C(=O)Oc1ccc(cc1)[N+]([O-])=O)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C27H24N2O9/c30-26(38-21-5-3-20(4-6-21)29(32)33)28-11-9-17(10-12-28)27(31,18-1-7-22-24(13-18)36-15-34-22)19-2-8-23-25(14-19)37-16-35-23/h1-8,13-14,17,31H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563791
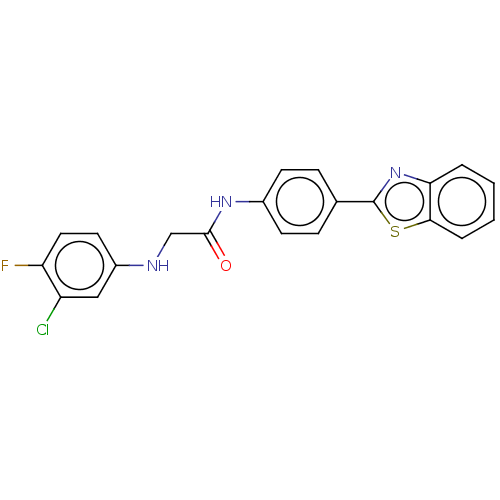
(CHEMBL4778803)Show SMILES Fc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222242
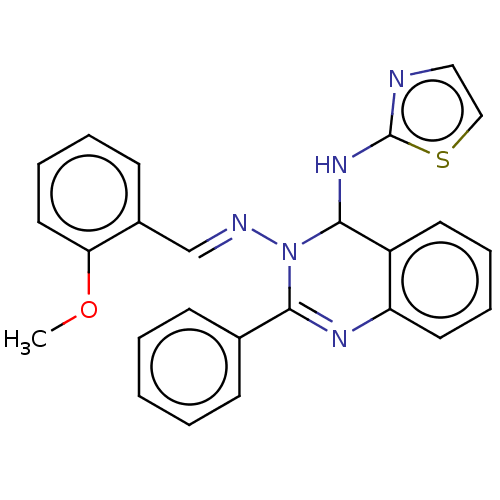
(N3-(2-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nccs2)c2ccccc2N=C1c1ccccc1 |c:27| Show InChI InChI=1S/C25H21N5OS/c1-31-22-14-8-5-11-19(22)17-27-30-23(18-9-3-2-4-10-18)28-21-13-7-6-12-20(21)24(30)29-25-26-15-16-32-25/h2-17,24H,1H3,(H,26,29)/b27-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222256
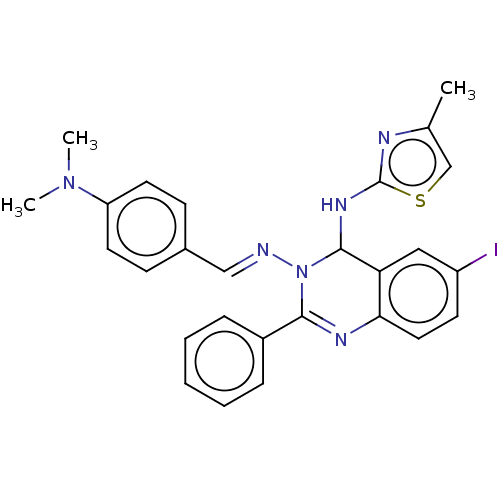
(N3-(4-(Dimethylamino)-benzylidene)-6-iodo-N4-(4-me...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nc(C)cs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:27| Show InChI InChI=1S/C27H25IN6S/c1-18-17-35-27(30-18)32-26-23-15-21(28)11-14-24(23)31-25(20-7-5-4-6-8-20)34(26)29-16-19-9-12-22(13-10-19)33(2)3/h4-17,26H,1-3H3,(H,30,32)/b29-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222251
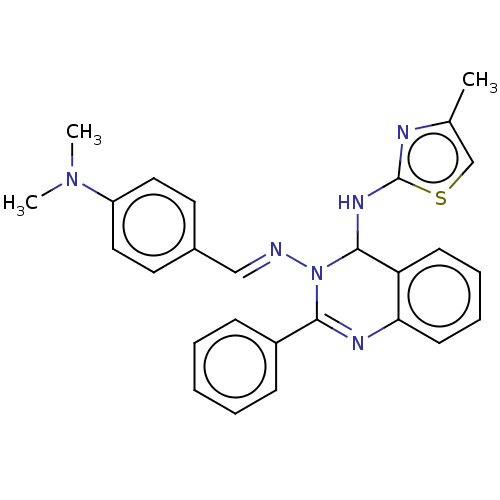
(N3-(4-(Dimethylamino)-benzylidene)-N4-(4-methylthi...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nc(C)cs3)c3ccccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C27H26N6S/c1-19-18-34-27(29-19)31-26-23-11-7-8-12-24(23)30-25(21-9-5-4-6-10-21)33(26)28-17-20-13-15-22(16-14-20)32(2)3/h4-18,26H,1-3H3,(H,29,31)/b28-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222253

(N3-Benzylidene-6-iodo-N4-(4-methylthiazol-2-yl)-2-...)Show SMILES Cc1csc(NC2N(\N=C\c3ccccc3)C(=Nc3ccc(I)cc23)c2ccccc2)n1 |c:17| Show InChI InChI=1S/C25H20IN5S/c1-17-16-32-25(28-17)30-24-21-14-20(26)12-13-22(21)29-23(19-10-6-3-7-11-19)31(24)27-15-18-8-4-2-5-9-18/h2-16,24H,1H3,(H,28,30)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222246
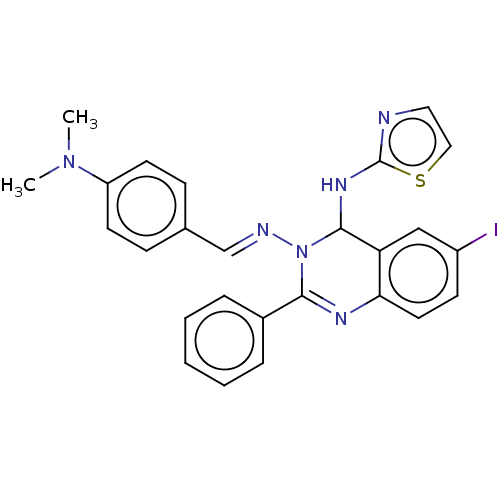
(N3-{4-(Dimethylamino)-benzylidene}-6-iodo-2-phenyl...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C26H23IN6S/c1-32(2)21-11-8-18(9-12-21)17-29-33-24(19-6-4-3-5-7-19)30-23-13-10-20(27)16-22(23)25(33)31-26-28-14-15-34-26/h3-17,25H,1-2H3,(H,28,31)/b29-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50466848
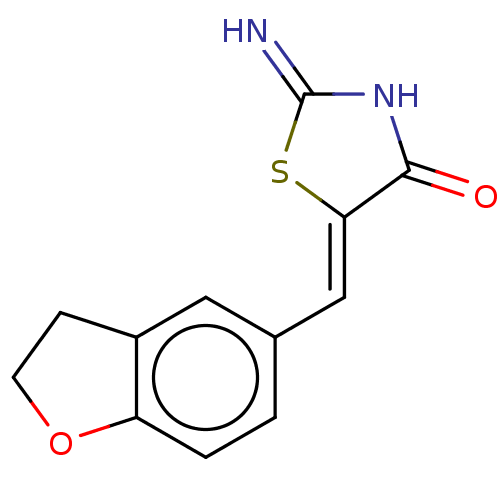
(CHEMBL4283751)Show InChI InChI=1S/C12H10N2O2S/c13-12-14-11(15)10(17-12)6-7-1-2-9-8(5-7)3-4-16-9/h1-2,5-6H,3-4H2,(H2,13,14,15)/b10-6- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563794
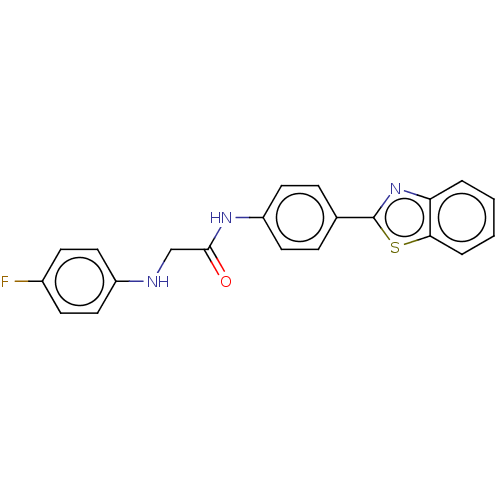
(CHEMBL4793668)Show SMILES Fc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563792
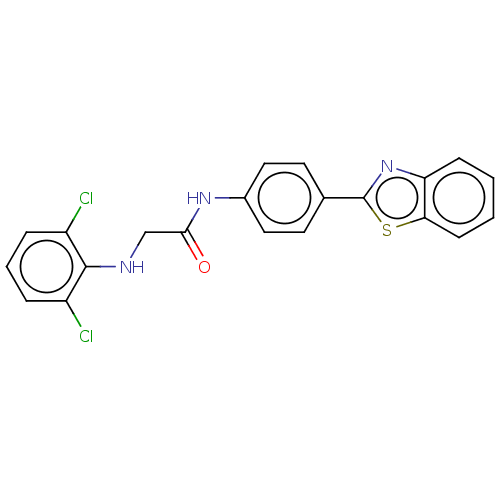
(CHEMBL4788586)Show SMILES Clc1cccc(Cl)c1NCC(=O)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222248
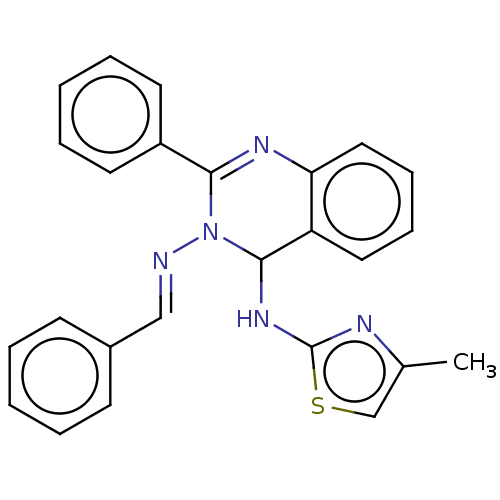
(N3-Benzylidene-N4-(4-methylthiazol-2-yl)-2-phenylq...)Show SMILES Cc1csc(NC2N(\N=C\c3ccccc3)C(=Nc3ccccc23)c2ccccc2)n1 |c:17| Show InChI InChI=1S/C25H21N5S/c1-18-17-31-25(27-18)29-24-21-14-8-9-15-22(21)28-23(20-12-6-3-7-13-20)30(24)26-16-19-10-4-2-5-11-19/h2-17,24H,1H3,(H,27,29)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222241
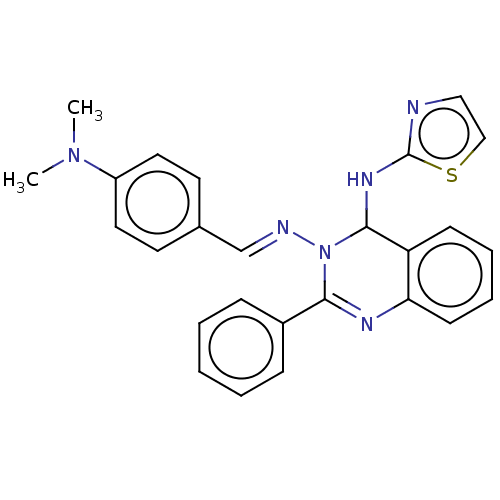
(N3-(4-(Dimethylamino)-benzylidene)-2-phenyl-N4-(th...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C26H24N6S/c1-31(2)21-14-12-19(13-15-21)18-28-32-24(20-8-4-3-5-9-20)29-23-11-7-6-10-22(23)25(32)30-26-27-16-17-33-26/h3-18,25H,1-2H3,(H,27,30)/b28-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563793

(CHEMBL4793997)Show SMILES Clc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563790

(CHEMBL4780294)Show SMILES Brc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447
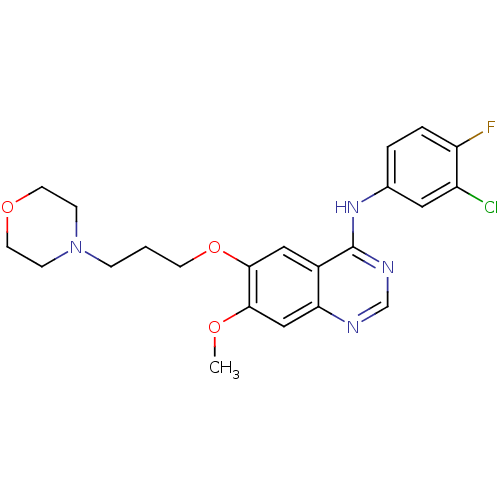
(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University
Curated by ChEMBL
| Assay Description
Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... |
Eur J Med Chem 126: 853-869 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.014
BindingDB Entry DOI: 10.7270/Q2CR5WKW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222243
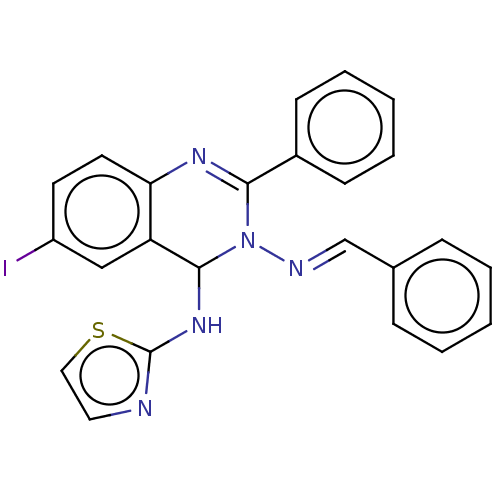
(N3-Benzylidene-6-iodo-2-phenyl-N4-(thiazol-2-yl)-q...)Show SMILES Ic1ccc2N=C(N(\N=C\c3ccccc3)C(Nc3nccs3)c2c1)c1ccccc1 |c:5| Show InChI InChI=1S/C24H18IN5S/c25-19-11-12-21-20(15-19)23(29-24-26-13-14-31-24)30(27-16-17-7-3-1-4-8-17)22(28-21)18-9-5-2-6-10-18/h1-16,23H,(H,26,29)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222238
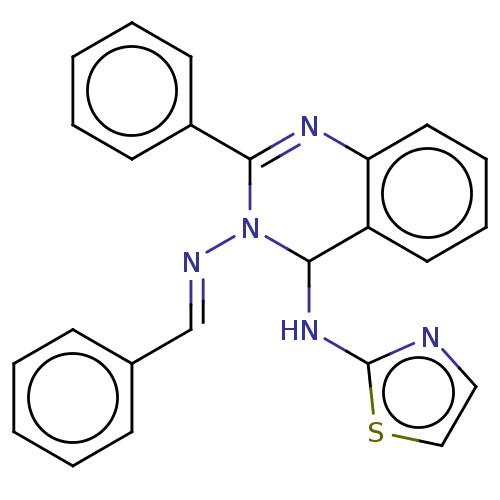
(N3-Benzylidene-2-phenyl-N4-(thiazol-2-yl)-quinazol...)Show SMILES N(C1N(\N=C\c2ccccc2)C(=Nc2ccccc12)c1ccccc1)c1nccs1 |c:12| Show InChI InChI=1S/C24H19N5S/c1-3-9-18(10-4-1)17-26-29-22(19-11-5-2-6-12-19)27-21-14-8-7-13-20(21)23(29)28-24-25-15-16-30-24/h1-17,23H,(H,25,28)/b26-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563801
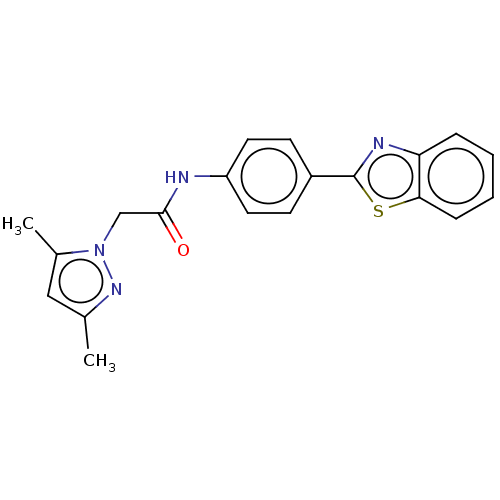
(CHEMBL4779678)Show SMILES Cc1cc(C)n(CC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563802
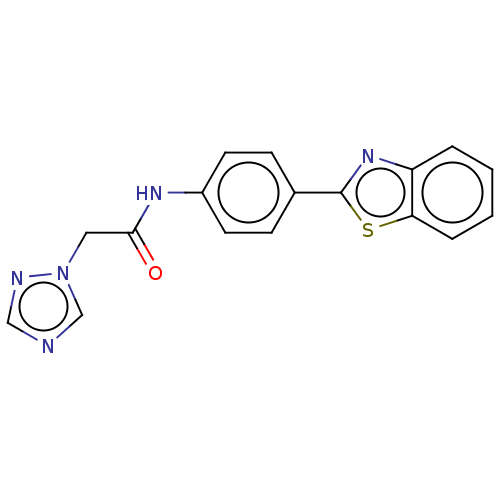
(CHEMBL4793265) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
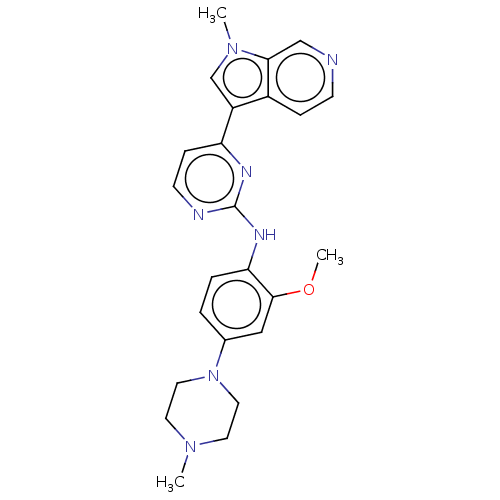
(Homo sapiens (Human)) | BDBM50081174
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Presenilin-1
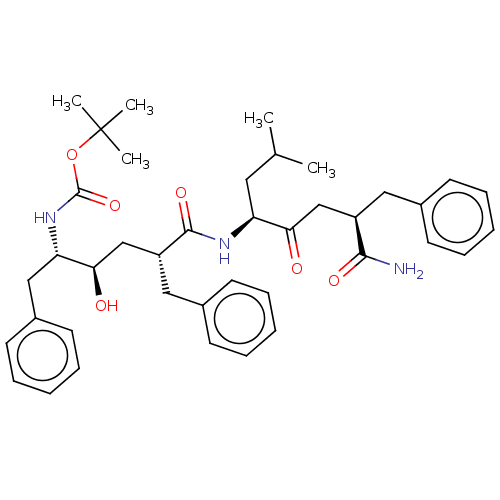
(Homo sapiens) | CHEMBL5283881
| MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081174

(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
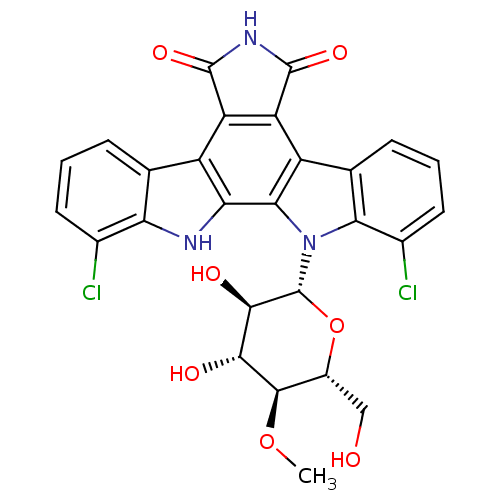
(Homo sapiens (Human)) | BDBM50162287
((Rebeccamycin)1,11-dichloro-12-(3,4-dihydroxy-6-hy...)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/C27H21Cl2N3O7/c1-38-24-13(8-33)39-27(23(35)22(24)34)32-20-10(5-3-7-12(20)29)15-17-16(25(36)31-26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)32/h2-7,13,22-24,27,30,33-35H,8H2,1H3,(H,31,36,37)/t13-,22-,23-,24-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50414921

(CHEMBL570812)Show SMILES COc1nn(-c2ccc(NC(=O)OCc3ccccc3)c(C)c2)c(=O)o1 Show InChI InChI=1S/C18H17N3O5/c1-12-10-14(21-18(23)26-17(20-21)24-2)8-9-15(12)19-16(22)25-11-13-6-4-3-5-7-13/h3-10H,11H2,1-2H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
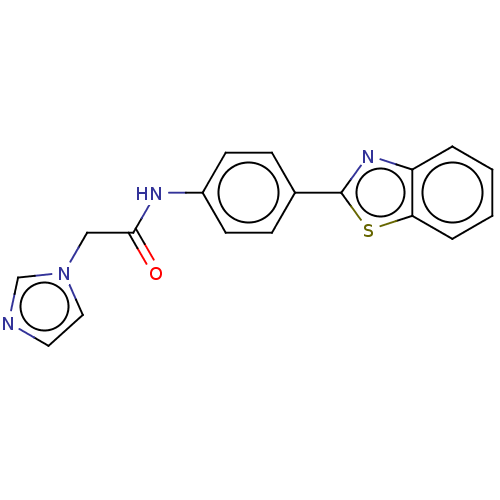
(Homo sapiens (Human)) | BDBM50563803
(CHEMBL4797565) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University
Curated by ChEMBL
| Assay Description
Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method |
Eur J Med Chem 126: 853-869 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.014
BindingDB Entry DOI: 10.7270/Q2CR5WKW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data