

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221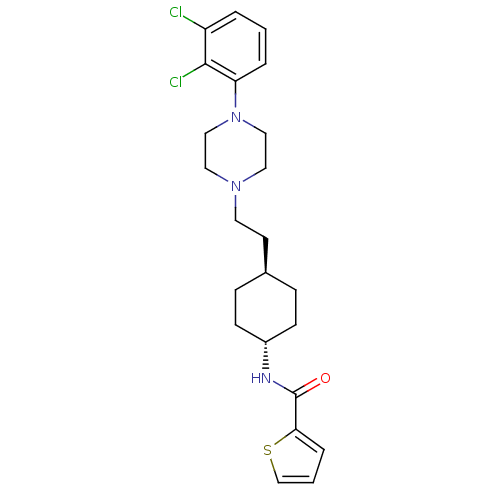 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063292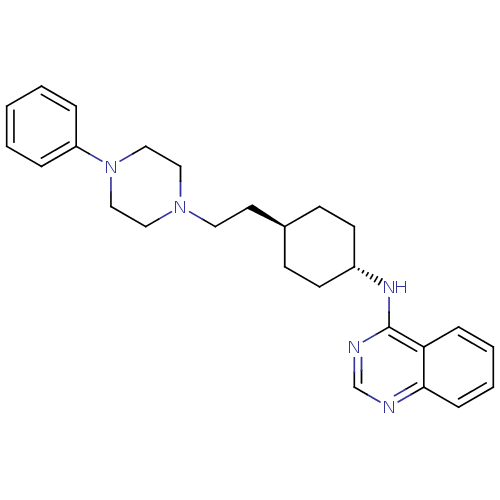 (CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286859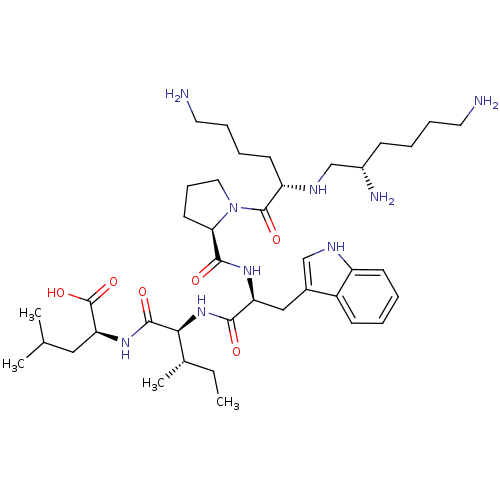 (2-{2-[(S)-2-({1-[(S)-6-Amino-2-((S)-(S)-2,6-diamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50123626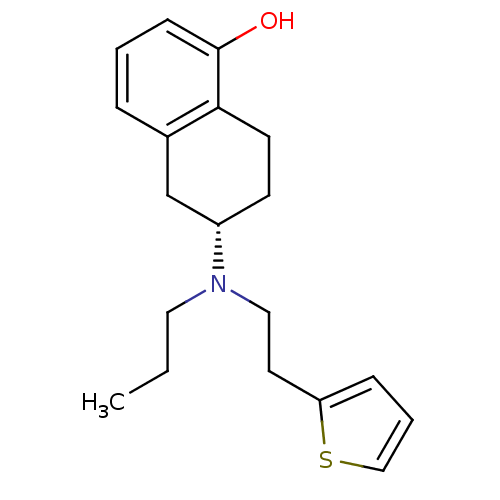 ((S)-6-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50240339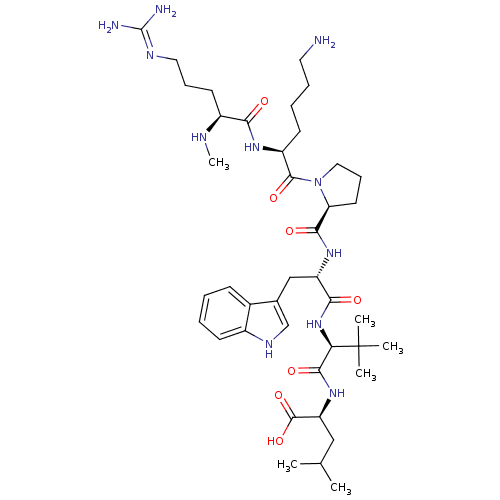 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for its binding affinity against Neurotensin Receptor after peripheral administration | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- prazosin binding against Alpha-1A adrenergic receptor from rat submaxillary gland | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343274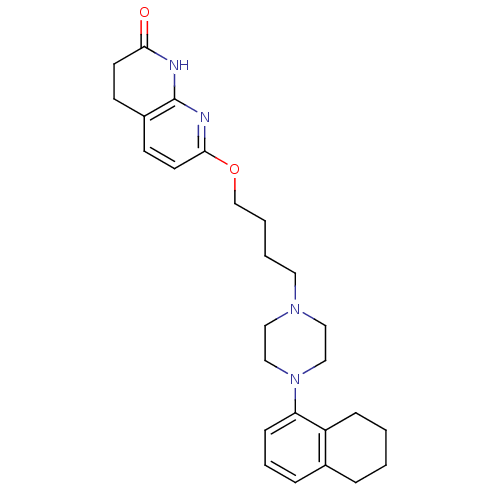 (7-(4-(4-(5,6,7,8-tetrahydronaphthalen-1-yl)piperaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286864 ((S)-2-{(S)-2-[(S)-2-({(R)-1-[5-Guanidino-2-(3-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343260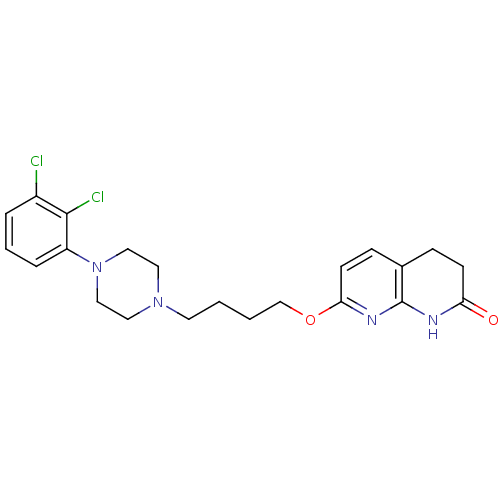 (7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343277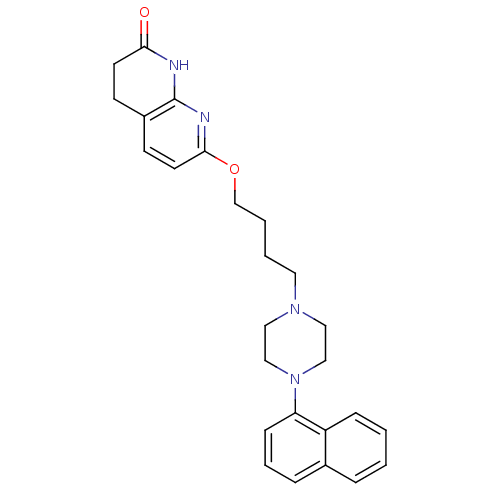 (7-(4-(4-(naphthalen-1-yl)piperazin-1-yl)butoxy)-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279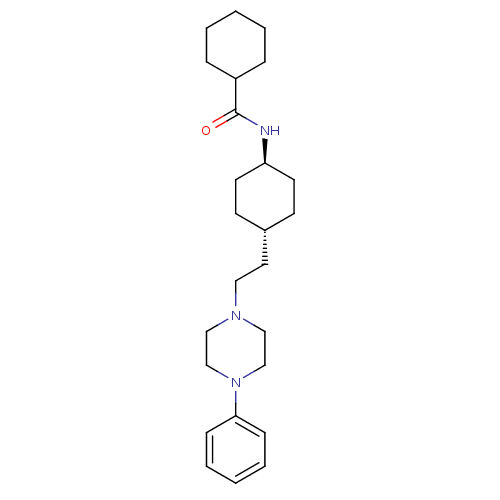 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010621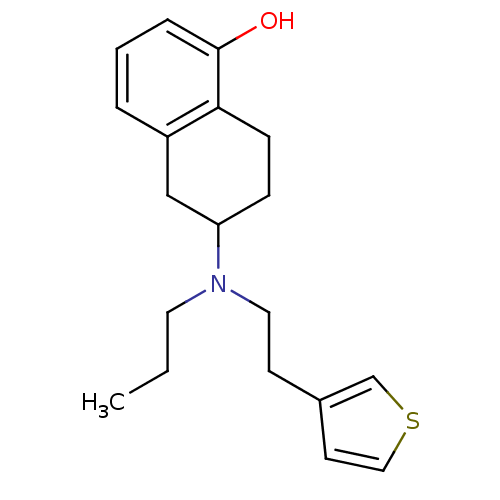 (6-[Propyl-(2-thiophen-3-yl-ethyl)-amino]-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50366427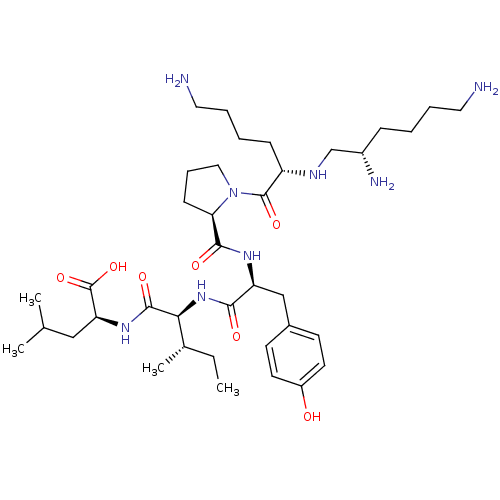 (CHEMBL1793865) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- prazosin binding against Alpha-1B adrenergic receptor from rat liver | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343273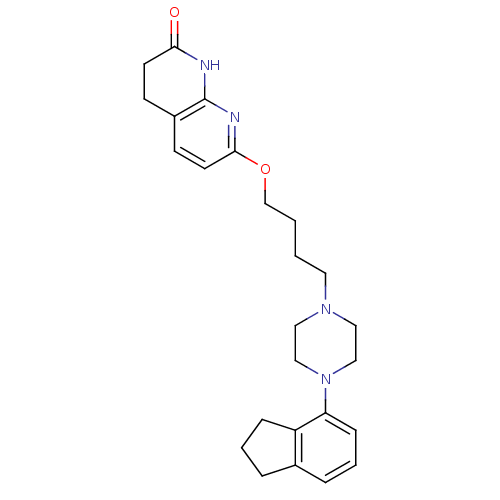 (7-(4-(4-(2,3-dihydro-1H-inden-4-yl)piperazin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343276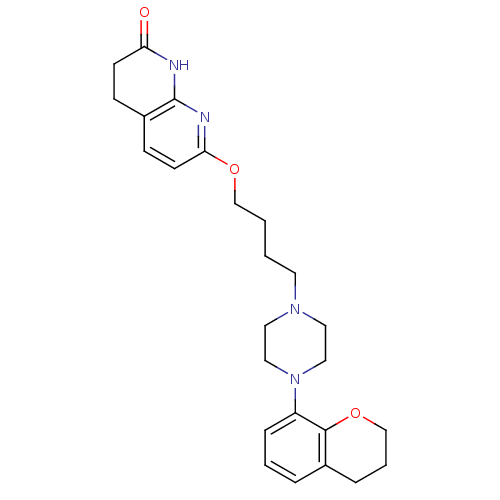 (7-(4-(4-(chroman-8-yl)piperazin-1-yl)butoxy)-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343283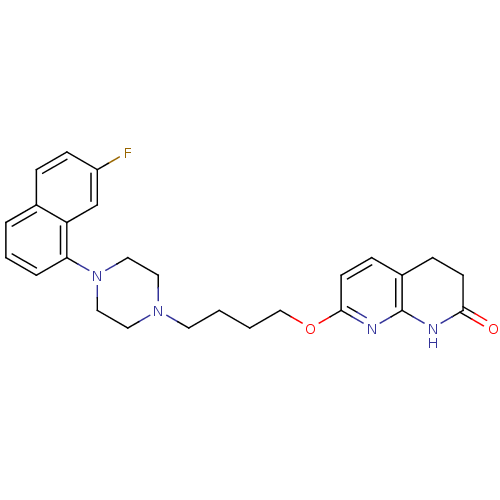 (7-(4-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested for [3H]-(+)-pentazocine binding to sigma-1 receptor in guinea pig brain membrane | J Med Chem 36: 3929-36 (1994) BindingDB Entry DOI: 10.7270/Q2C828CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343268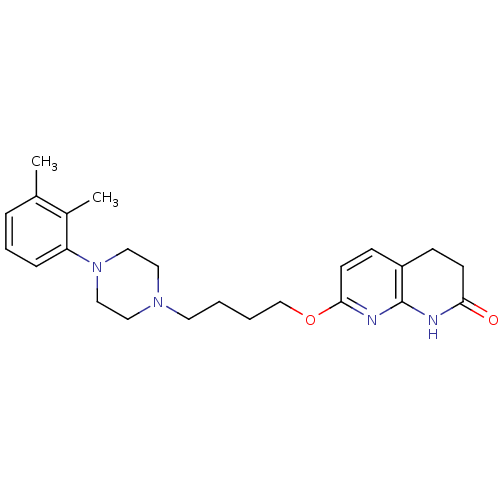 (7-(4-(4-(2,3-dimethylphenyl)piperazin-1-yl)butoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50052535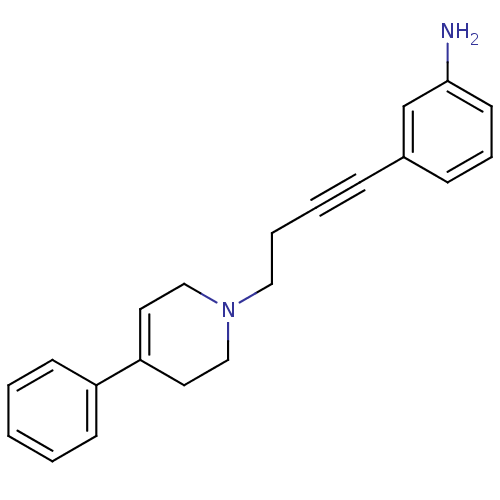 (3-[4-(4-Phenyl-3,6-dihydro-2H-pyridin-1-yl)-but-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 3179-87 (1996) Article DOI: 10.1021/jm950721m BindingDB Entry DOI: 10.7270/Q23N22GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286868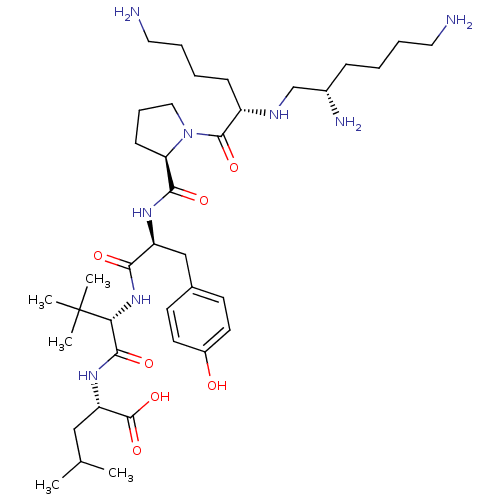 ((1S,2S,4S)-2-{2-[(S)-2-({1-[6-Amino-2-(2,6-diamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50002173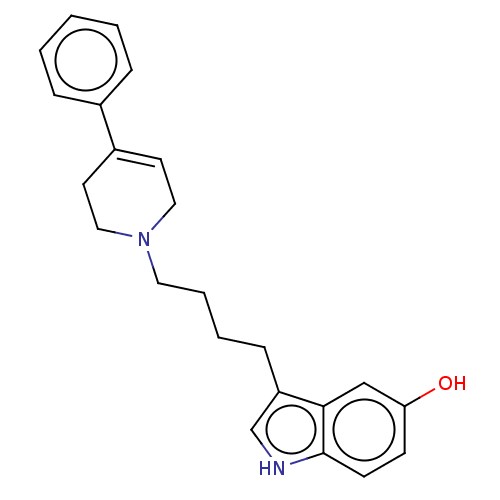 (3-(4-(3,6-dihydro-4-phenyl-1(2H)-pyridinyl)butyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 in CHO-K1 cells using [3H]-Spiperone as radioligand | J Med Chem 39: 3179-87 (1996) Article DOI: 10.1021/jm950721m BindingDB Entry DOI: 10.7270/Q23N22GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from CHO-K1 cell membranes expressing human dopamine receptor D2 | J Med Chem 36: 3929-36 (1994) BindingDB Entry DOI: 10.7270/Q2C828CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343266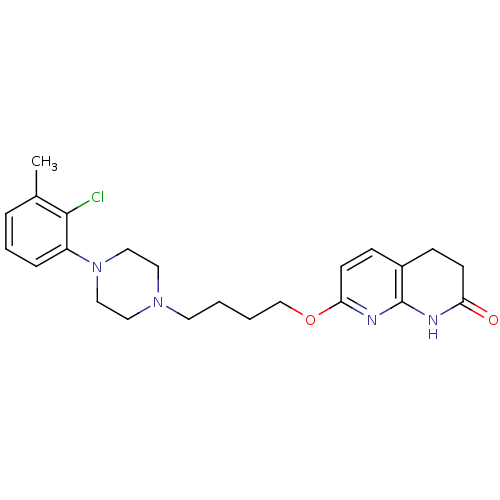 (7-(4-(4-(2-chloro-3-methylphenyl)piperazin-1-yl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50063281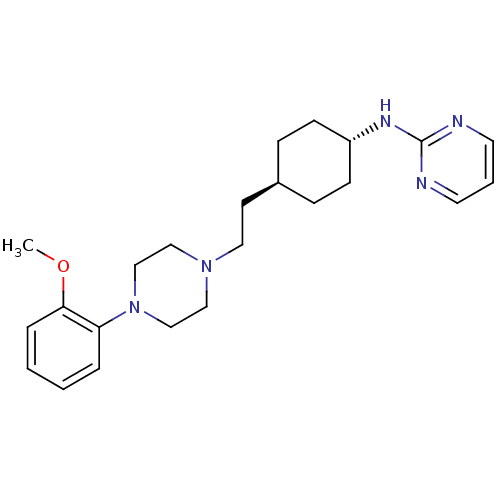 ((4-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The binding affinity of the compound was determined by measuring its ability to displace [3H]-8-OH-DPAT radioligand in 5-hydroxytryptamine 1A recepto... | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010289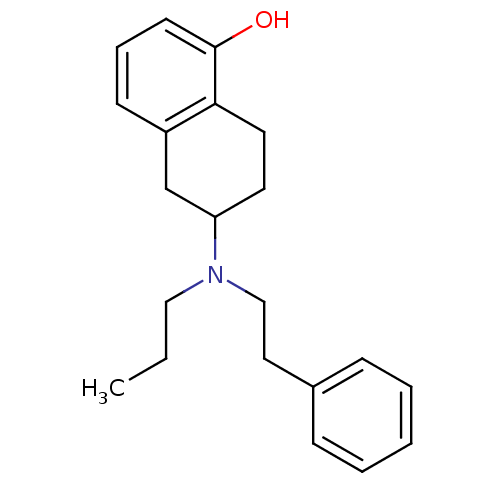 ((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054070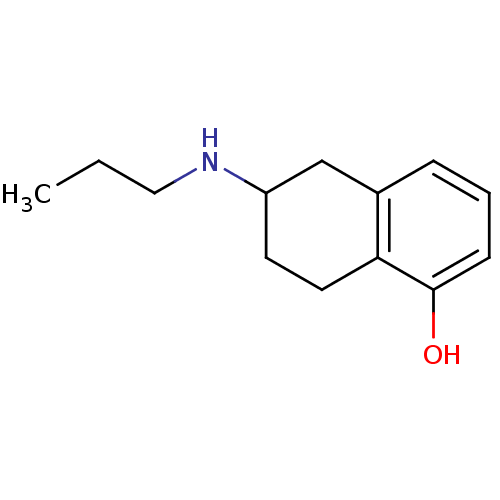 (6-Propylamino-5,6,7,8-tetrahydro-naphthalen-1-ol |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054067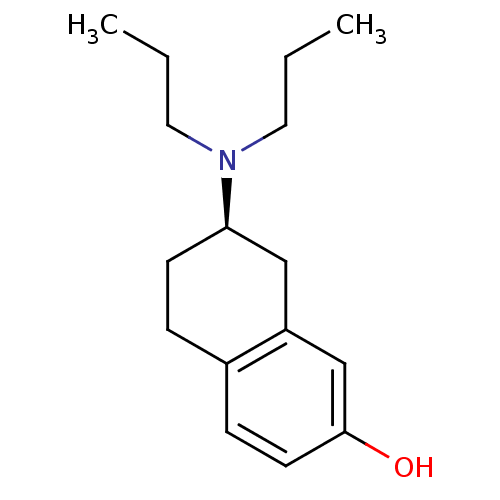 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290206 (CHEMBL78800 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50240845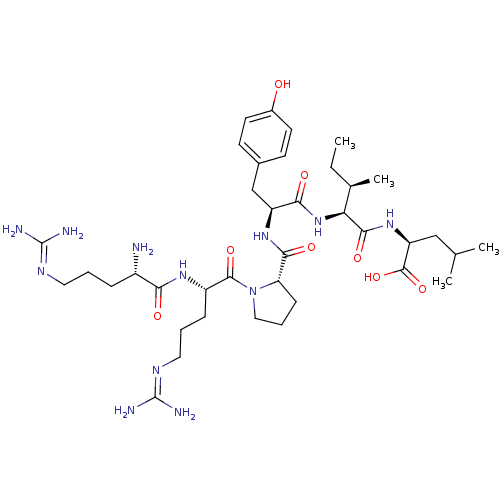 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50115277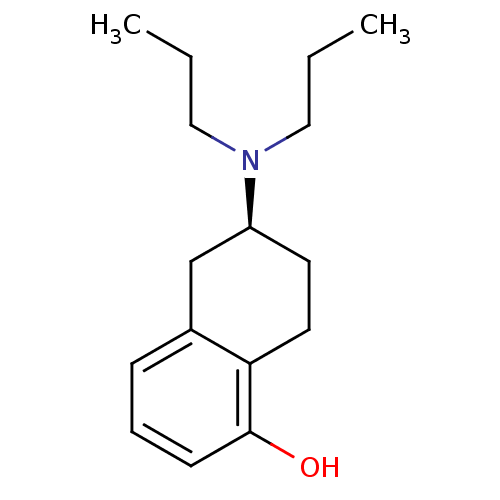 ((2R,3R)-6-Dipropylamino-5,6,7,8-tetrahydro-naphtha...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290225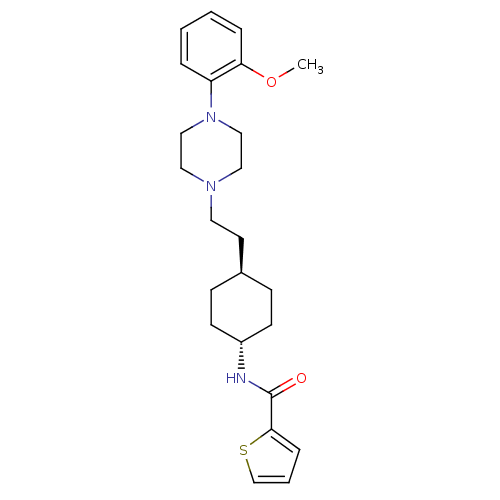 (CHEMBL78950 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290221 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054077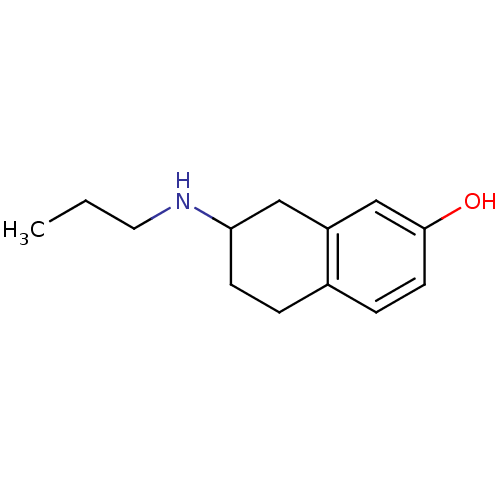 (7-Propylamino-5,6,7,8-tetrahydro-naphthalen-2-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054075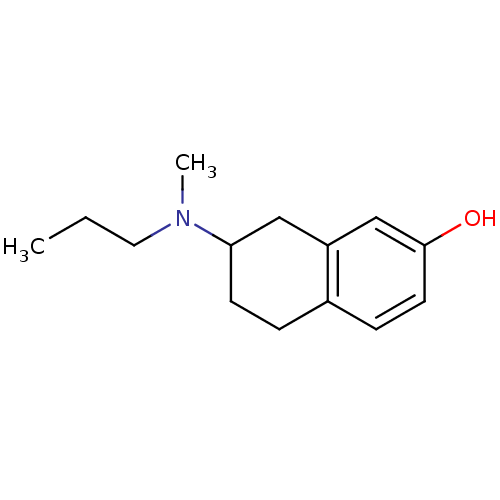 (7-(Methyl-propyl-amino)-5,6,7,8-tetrahydro-naphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343284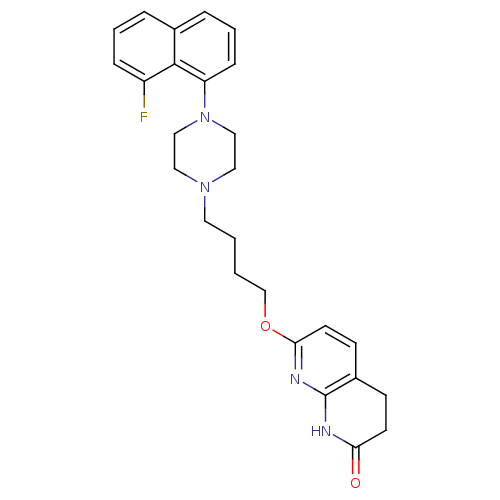 (7-(4-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052531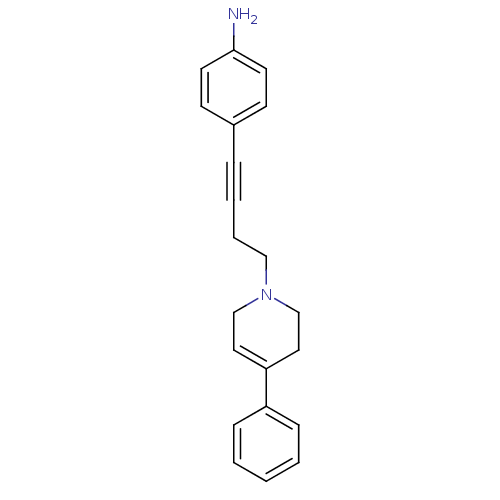 (4-[4-(4-Phenyl-3,6-dihydro-2H-pyridin-1-yl)-but-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinityagainst Dopamine receptor D2 in CHO-K1 cells using radioligand [3H]-NPA binding assay | J Med Chem 39: 3179-87 (1996) Article DOI: 10.1021/jm950721m BindingDB Entry DOI: 10.7270/Q23N22GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50343276 (7-(4-(4-(chroman-8-yl)piperazin-1-yl)butoxy)-3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human dopamine D2L receptor expressed in CHO cells | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054069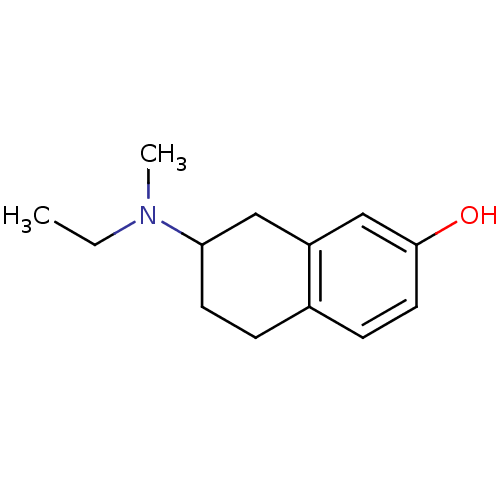 (7-(Ethyl-methyl-amino)-5,6,7,8-tetrahydro-naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054075 (7-(Methyl-propyl-amino)-5,6,7,8-tetrahydro-naphtha...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054082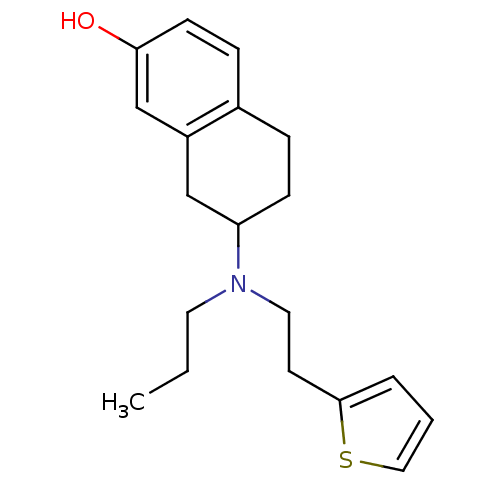 (7-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054070 (6-Propylamino-5,6,7,8-tetrahydro-naphthalen-1-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]spiperone binding on Dopamine receptor D3 expressed on CHO K-1 cells. | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054082 (7-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Binding affinity was evaluated by calculating competition for [3H]N-0437 binding on Dopamine receptor D2L of CHO K-1 cells | J Med Chem 39: 4233-7 (1996) Article DOI: 10.1021/jm960345l BindingDB Entry DOI: 10.7270/Q21C1XJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50343284 (7-(4-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50343275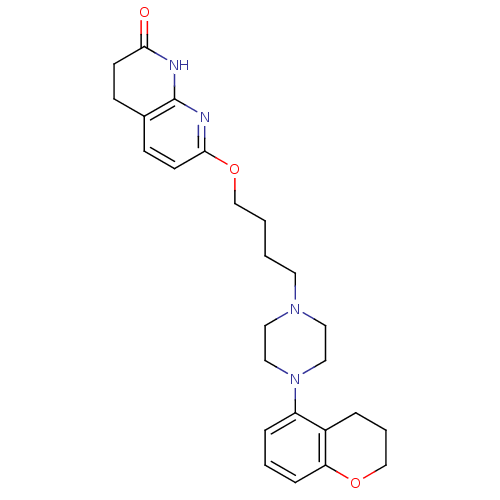 (7-(4-(4-(chroman-5-yl)piperazin-1-yl)butoxy)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50343259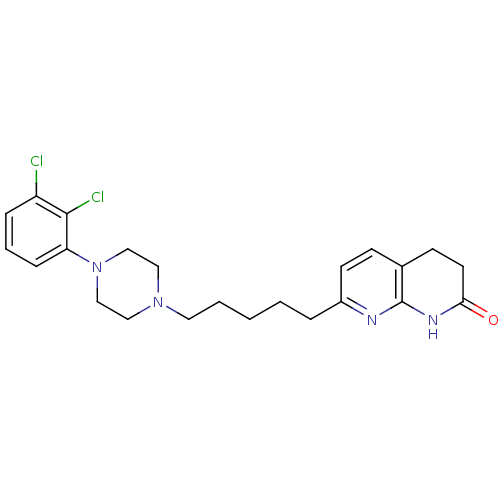 (7-(5-(4-(2,3-dichlorophenyl)piperazin-1-yl)pentyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 21: 2621-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.059 BindingDB Entry DOI: 10.7270/Q29K4BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063291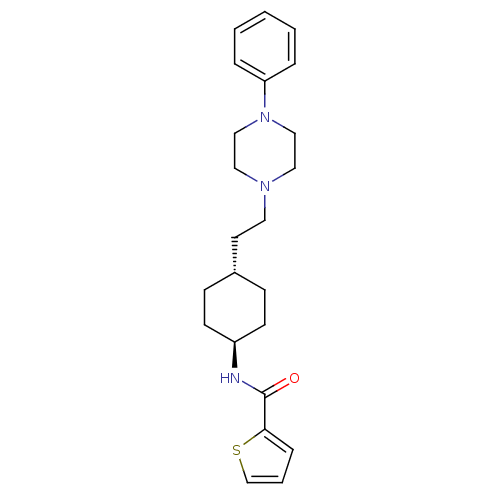 (CHEMBL78791 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 646 total ) | Next | Last >> |