

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin E (Homo sapiens (Human)) | BDBM912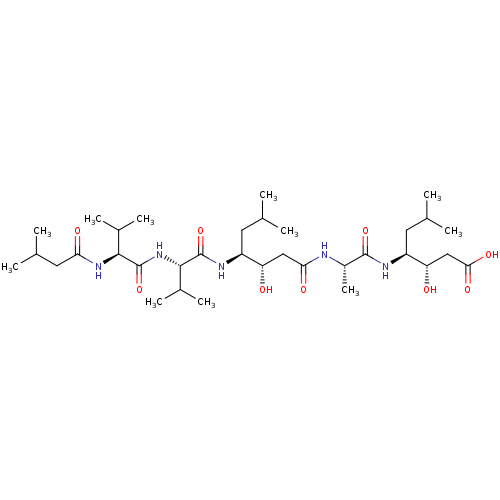 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin E using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins f... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of secreted cathepsin E in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of secreted cathepsin D in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50101331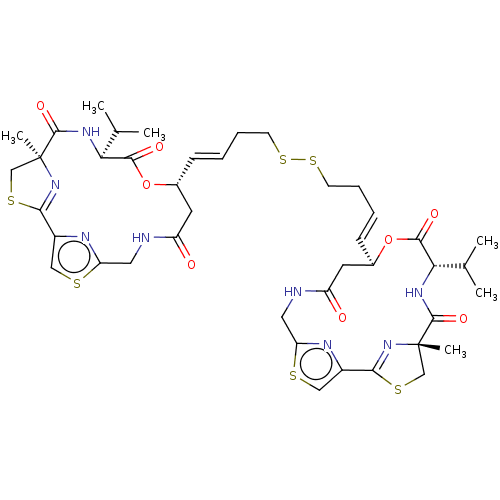 (CHEMBL3329621) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50101330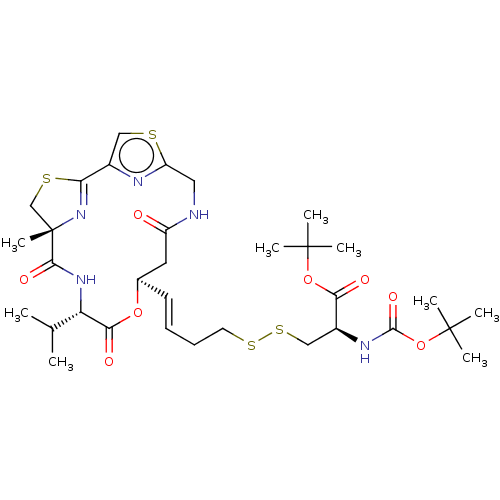 (CHEMBL3329622) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50101331 (CHEMBL3329621) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50101331 (CHEMBL3329621) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50101331 (CHEMBL3329621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50302109 (CHEMBL568553 | Grassystatin B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50101330 (CHEMBL3329622) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50101330 (CHEMBL3329622) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283681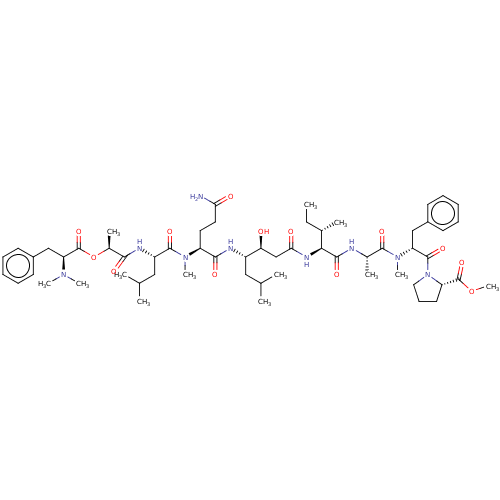 (CHEMBL4163078) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50101331 (CHEMBL3329621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50101330 (CHEMBL3329622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50101330 (CHEMBL3329622) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50302107 (CHEMBL567893 | Grassystatin A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC11 (unknown origin) incubated for 3 hrs in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283686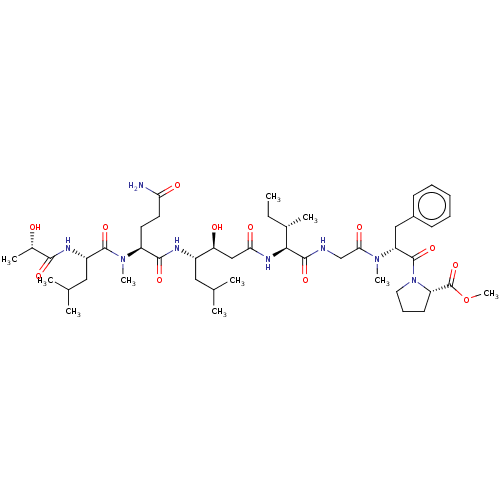 (CHEMBL4170992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50283681 (CHEMBL4163078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of cathepsin D in human MDA-MB-231 cell lysates using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated f... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50302109 (CHEMBL568553 | Grassystatin B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283684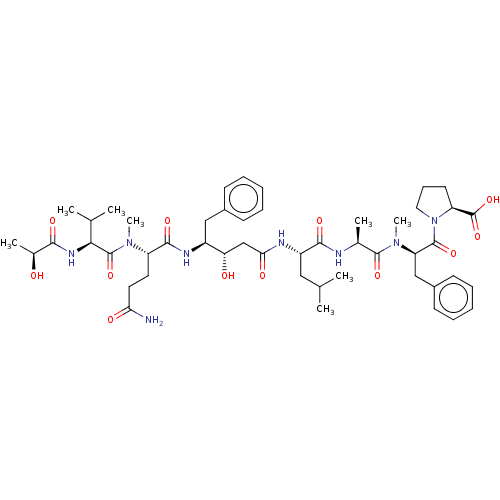 (CHEMBL4167618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50400221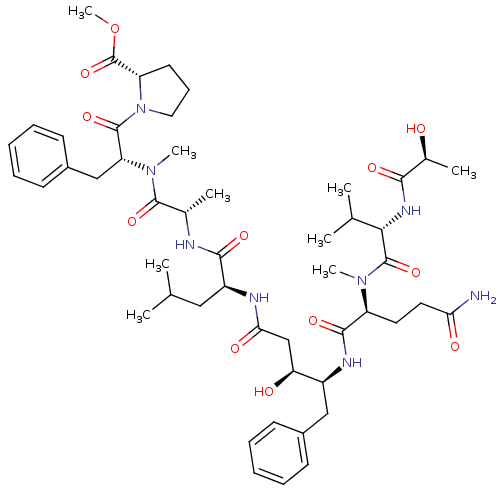 (CHEMBL2181015) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin E using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins f... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50288847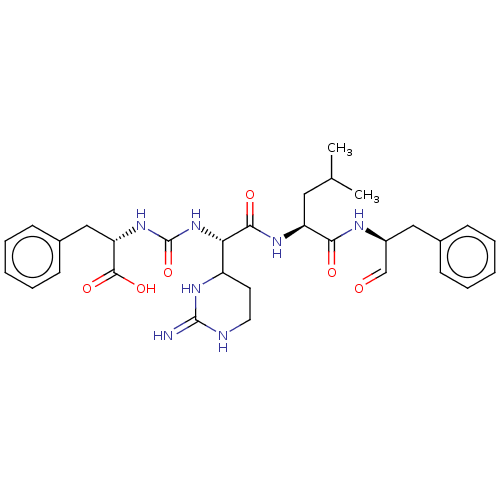 (Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of chymase (unknown origin) by fluorescence assay | J Med Chem 61: 6364-6378 (2018) Article DOI: 10.1021/acs.jmedchem.8b00885 BindingDB Entry DOI: 10.7270/Q2N87D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283685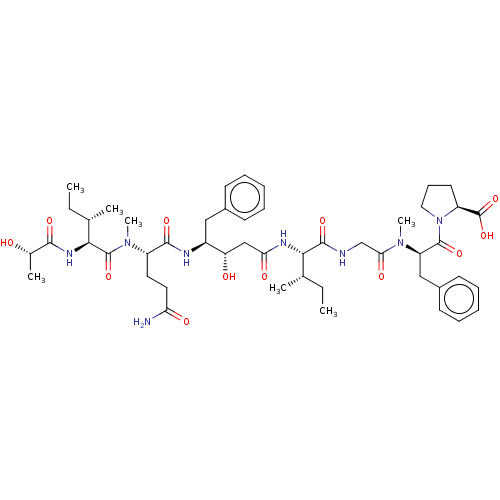 (CHEMBL4159706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50178261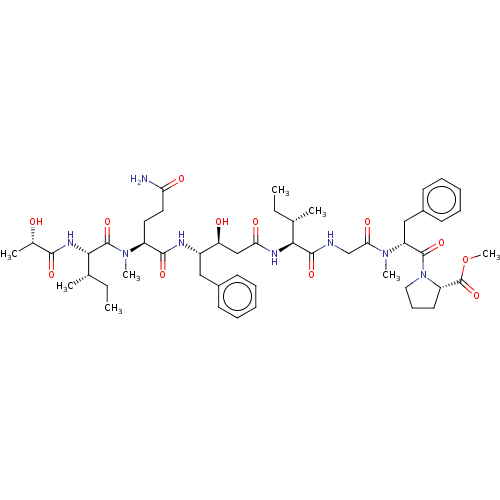 (CHEMBL3813928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin E using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins f... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50302107 (CHEMBL567893 | Grassystatin A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283683 (CHEMBL4173516) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50020912 (Largazole Thiol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283682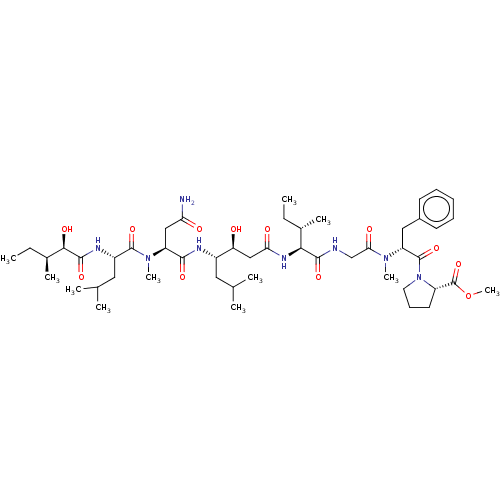 (CHEMBL4174823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50283684 (CHEMBL4167618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50283681 (CHEMBL4163078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50400221 (CHEMBL2181015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50178261 (CHEMBL3813928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50283685 (CHEMBL4159706) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50101331 (CHEMBL3329621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay | ACS Med Chem Lett 5: 905-10 (2014) Article DOI: 10.1021/ml500170r BindingDB Entry DOI: 10.7270/Q2RV0QFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50400221 (CHEMBL2181015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using peptide substrate by time-course measurement-based fluorescence analysis | Bioorg Med Chem 24: 3276-82 (2016) Article DOI: 10.1016/j.bmc.2016.04.062 BindingDB Entry DOI: 10.7270/Q2QZ2CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283683 (CHEMBL4173516) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of secreted cathepsin E in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50283681 (CHEMBL4163078) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of secreted cathepsin E in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... | J Nat Prod 80: 2969-2986 (2017) Article DOI: 10.1021/acs.jnatprod.7b00551 BindingDB Entry DOI: 10.7270/Q2JS9SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |