

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50226709 (CHEMBL3143111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021750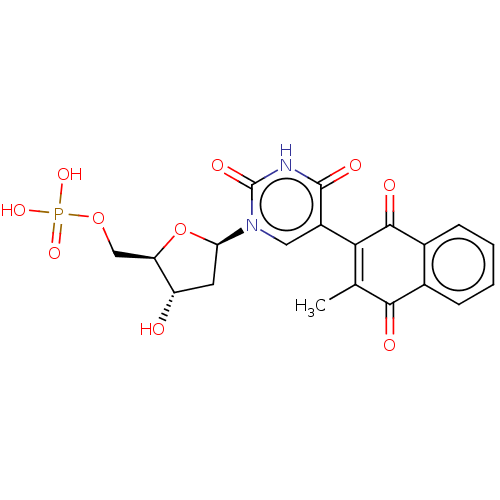 (CHEMBL3143104 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021748 (CHEMBL3143138 | Phosphoric acid mono-{5-[5-(4,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021746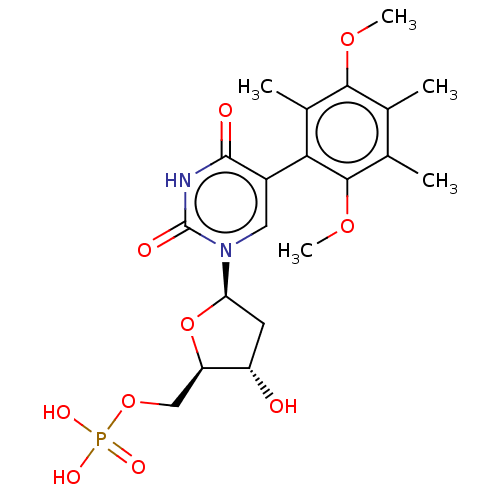 (CHEMBL3143107 | Phosphoric acid mono-{5-[5-(2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50021750 (CHEMBL3143104 | Phosphoric acid mono-{3-hydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021749 (CHEMBL3144392 | Phosphoric acid mono-{5-[5-(3,6-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021751 (CHEMBL3143106 | Phosphoric acid mono-{5-[5-(1,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50024666 ((1,4-Dimethoxy-3-methyl-naphthalen-2-yl)-phosphoni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity towards L. casei thymidylate synthase was determined | J Med Chem 30: 1705-6 (1987) BindingDB Entry DOI: 10.7270/Q23F4NNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50021752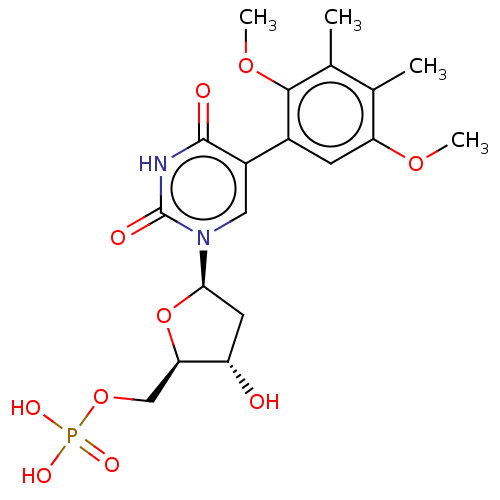 (CHEMBL3143105 | Phosphoric acid mono-{5-[5-(2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from L. casei was determined and expressed as inhibition constant (Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50021748 (CHEMBL3143138 | Phosphoric acid mono-{5-[5-(4,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50226709 (CHEMBL3143111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the thymidylate synthase from murine leukemia L1210 cells was determined and expressed as inhibition constant(Ki) | J Med Chem 30: 409-19 (1987) BindingDB Entry DOI: 10.7270/Q2NP23DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||