 Found 113 hits with Last Name = 'al-sha''er' and Initial = 'ma'
Found 113 hits with Last Name = 'al-sha''er' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25077
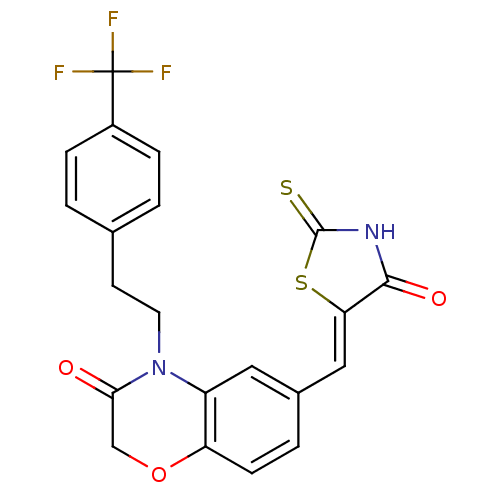
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H15F3N2O3S2/c22-21(23,24)14-4-1-12(2-5-14)7-8-26-15-9-13(3-6-16(15)29-11-18(26)27)10-17-19(28)25-20(30)31-17/h1-6,9-10H,7-8,11H2,(H,25,28,30)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25073
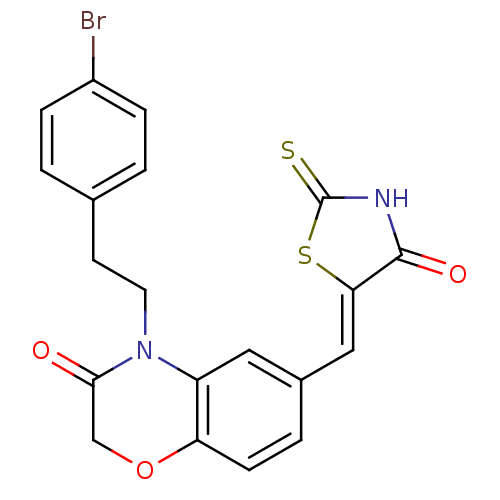
(4-[2-(4-bromophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Brc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15BrN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25061
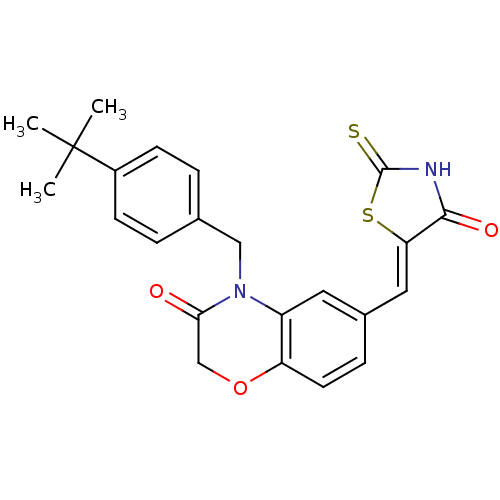
(4-[(4-tert-butylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES CC(C)(C)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C23H22N2O3S2/c1-23(2,3)16-7-4-14(5-8-16)12-25-17-10-15(6-9-18(17)28-13-20(25)26)11-19-21(27)24-22(29)30-19/h4-11H,12-13H2,1-3H3,(H,24,27,29)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25068
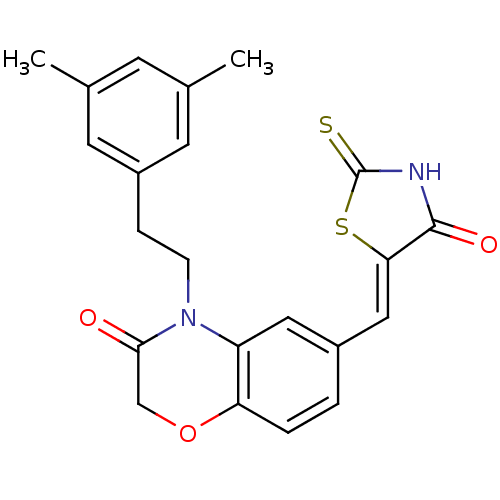
(4-[2-(3,5-dimethylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Cc1cc(C)cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C22H20N2O3S2/c1-13-7-14(2)9-16(8-13)5-6-24-17-10-15(3-4-18(17)27-12-20(24)25)11-19-21(26)23-22(28)29-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,28)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25066
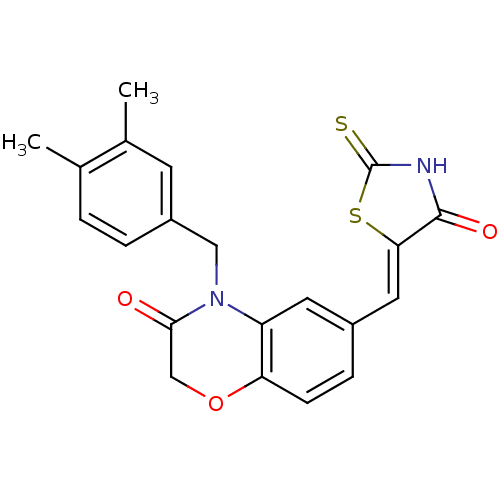
(4-[(3,4-dimethylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1C Show InChI InChI=1S/C21H18N2O3S2/c1-12-3-4-15(7-13(12)2)10-23-16-8-14(5-6-17(16)26-11-19(23)24)9-18-20(25)22-21(27)28-18/h3-9H,10-11H2,1-2H3,(H,22,25,27)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25080
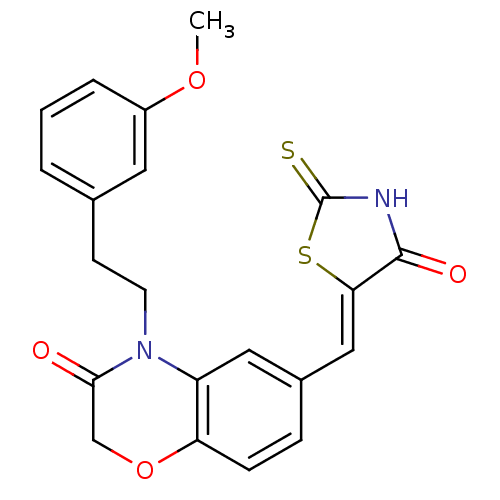
(4-[2-(3-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-4-2-3-13(9-15)7-8-23-16-10-14(5-6-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25069
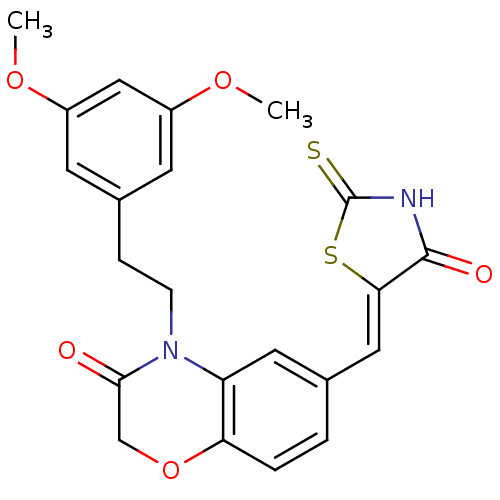
(4-[2-(3,5-dimethoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-...)Show SMILES COc1cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc(OC)c1 Show InChI InChI=1S/C22H20N2O5S2/c1-27-15-7-14(8-16(11-15)28-2)5-6-24-17-9-13(3-4-18(17)29-12-20(24)25)10-19-21(26)23-22(30)31-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,30)/b19-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25075
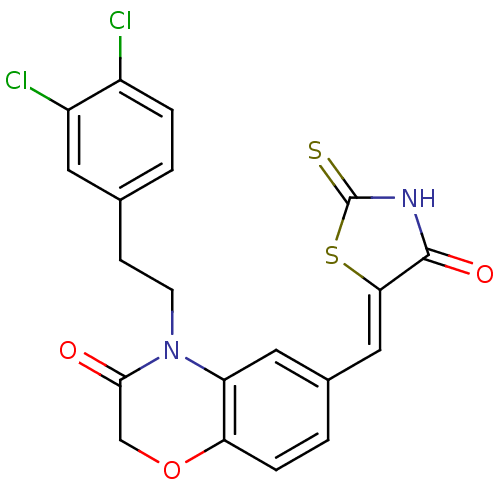
(4-[2-(3,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1Cl Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25078
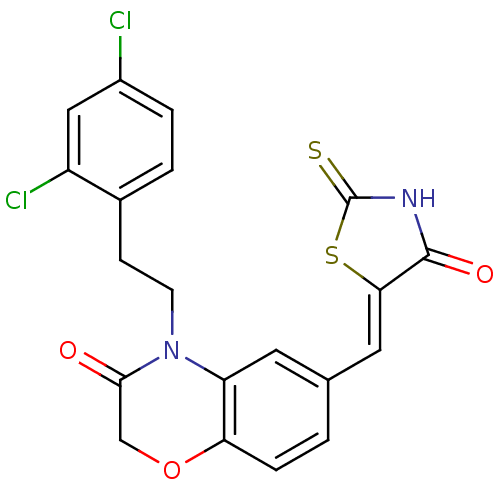
(4-[2-(2,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-2-12(14(22)9-13)5-6-24-15-7-11(1-4-16(15)27-10-18(24)25)8-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25074
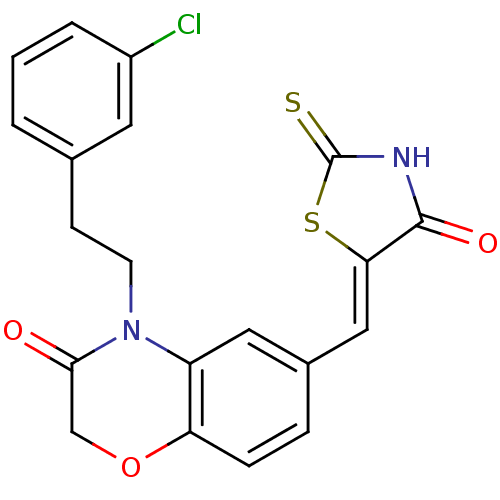
(4-[2-(3-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-3-1-2-12(8-14)6-7-23-15-9-13(4-5-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-5,8-10H,6-7,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189741
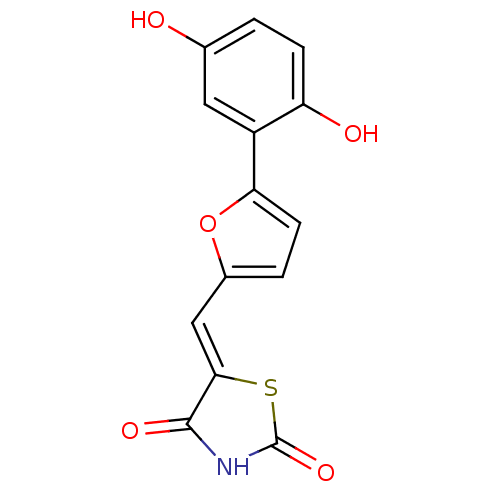
(5-[5-(2,5-dihydroxyphenyl)furan-2-ylmethylene]thia...)Show InChI InChI=1S/C14H9NO5S/c16-7-1-3-10(17)9(5-7)11-4-2-8(20-11)6-12-13(18)15-14(19)21-12/h1-6,16-17H,(H,15,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25070
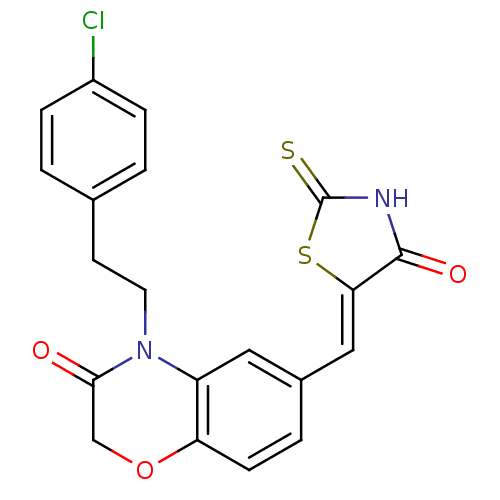
(4-[2-(4-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25081
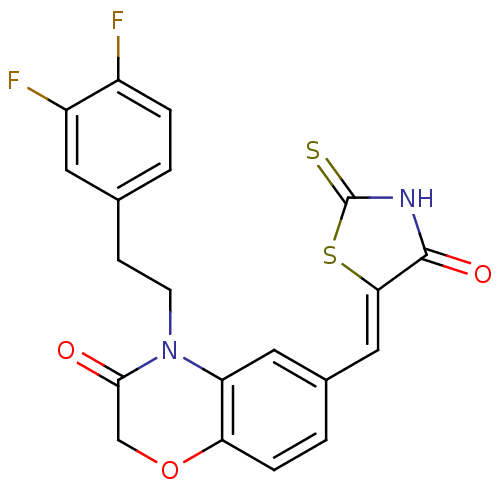
(4-[2-(3,4-difluorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Fc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1F Show InChI InChI=1S/C20H14F2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189760
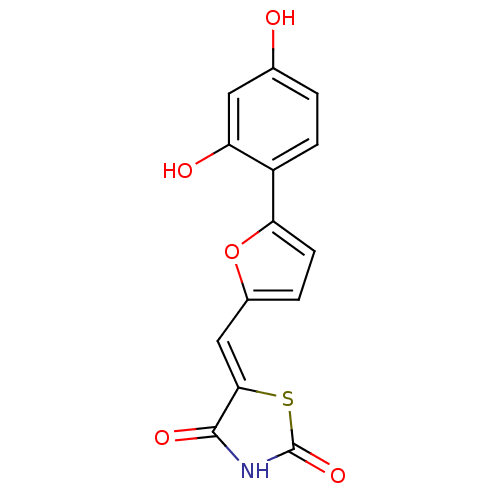
(5-[5-(2,4-dihydroxyphenyl)furan-2-ylmethylene]thia...)Show InChI InChI=1S/C14H9NO5S/c16-7-1-3-9(10(17)5-7)11-4-2-8(20-11)6-12-13(18)15-14(19)21-12/h1-6,16-17H,(H,15,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25079
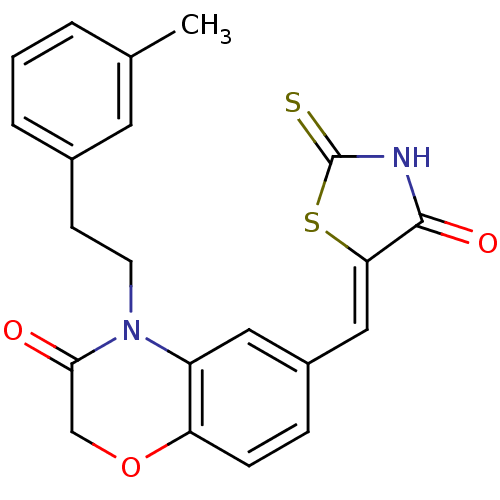
(4-[2-(3-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-3-2-4-14(9-13)7-8-23-16-10-15(5-6-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25071
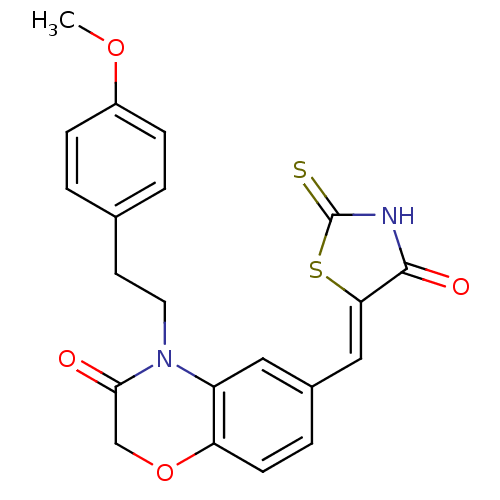
(4-[2-(4-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-5-2-13(3-6-15)8-9-23-16-10-14(4-7-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50055033
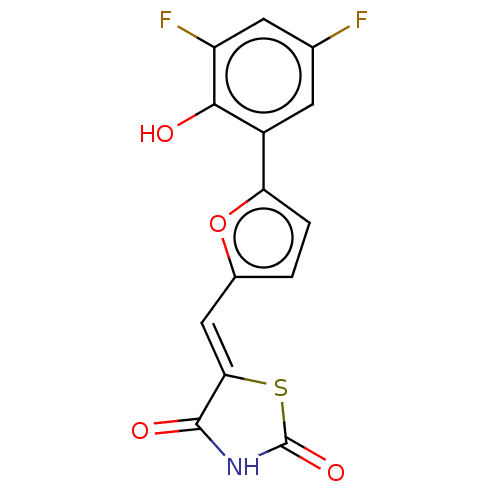
(CHEMBL3325884)Show InChI InChI=1S/C14H7F2NO4S/c15-6-3-8(12(18)9(16)4-6)10-2-1-7(21-10)5-11-13(19)17-14(20)22-11/h1-5,18H,(H,17,19,20)/b11-5- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM5655

(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...)Show SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human GST-CDK1/cyclin B1 expressed in baculovirus using [gamma-33P]ATP after 45 mins by liquid scintillation counting |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25062
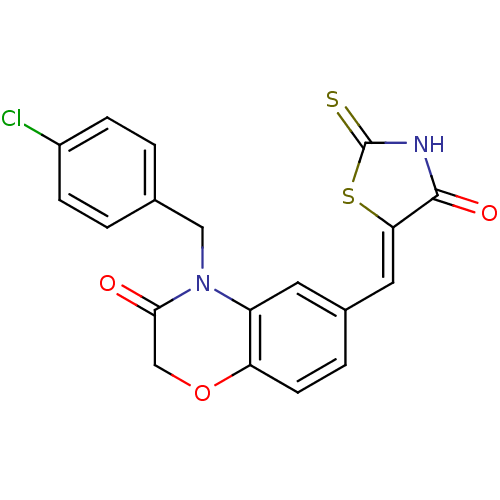
(4-[(4-chlorophenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Clc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C19H13ClN2O3S2/c20-13-4-1-11(2-5-13)9-22-14-7-12(3-6-15(14)25-10-17(22)23)8-16-18(24)21-19(26)27-16/h1-8H,9-10H2,(H,21,24,26)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189750
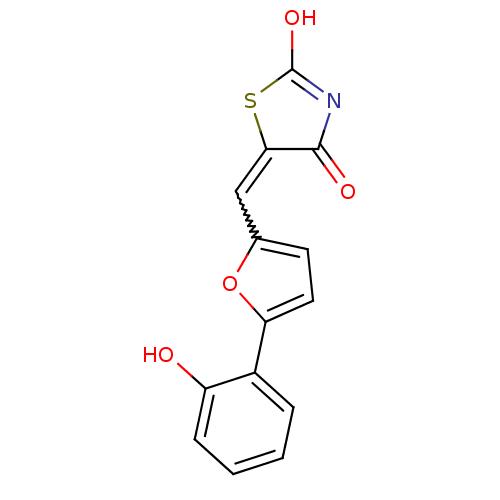
(5-[5-(2-hydroxyphenyl)furan-2-ylmethylene]thiazoli...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccccc1O |w:7.8,t:1| Show InChI InChI=1S/C14H9NO4S/c16-10-4-2-1-3-9(10)11-6-5-8(19-11)7-12-13(17)15-14(18)20-12/h1-7,16H,(H,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189744
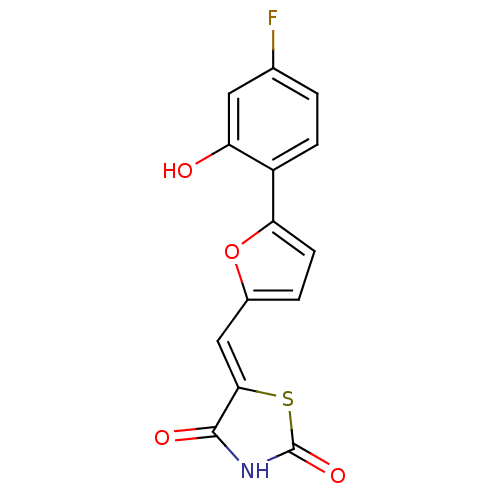
((Z)-5-((5-(4-fluoro-2-hydroxyphenyl)furan-2-yl)met...)Show InChI InChI=1S/C14H8FNO4S/c15-7-1-3-9(10(17)5-7)11-4-2-8(20-11)6-12-13(18)16-14(19)21-12/h1-6,17H,(H,16,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189743
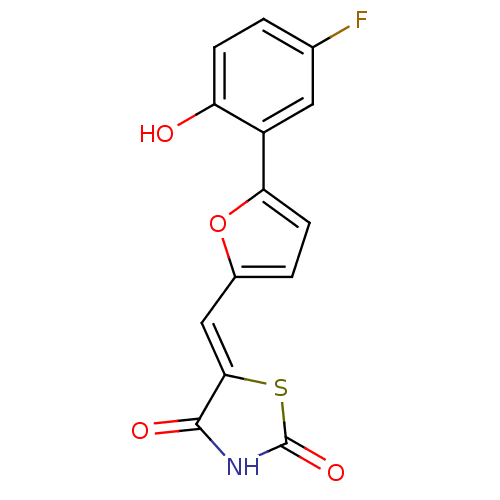
(5-[5-(5-fluoro-2-hydroxyphenyl)furan-2-ylmethylene...)Show InChI InChI=1S/C14H8FNO4S/c15-7-1-3-10(17)9(5-7)11-4-2-8(20-11)6-12-13(18)16-14(19)21-12/h1-6,17H,(H,16,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25072
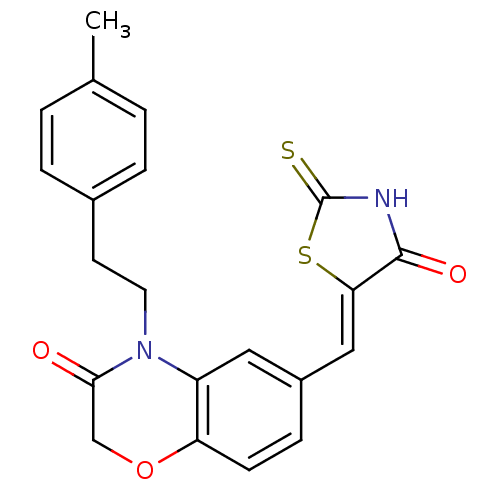
(4-[2-(4-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-2-4-14(5-3-13)8-9-23-16-10-15(6-7-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189745
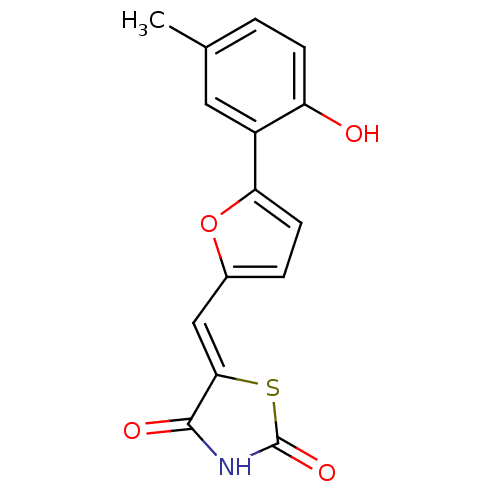
(5-[5-(2-hydroxy-5-methylphenyl)furan-2-ylmethylene...)Show InChI InChI=1S/C15H11NO4S/c1-8-2-4-11(17)10(6-8)12-5-3-9(20-12)7-13-14(18)16-15(19)21-13/h2-7,17H,1H3,(H,16,18,19)/b13-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189751
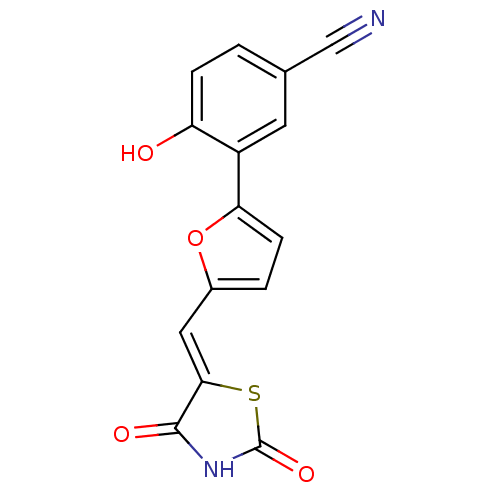
(3-[5-(2,4-dioxothiazolidin-5-ylidenemethyl)furan-2...)Show InChI InChI=1S/C15H8N2O4S/c16-7-8-1-3-11(18)10(5-8)12-4-2-9(21-12)6-13-14(19)17-15(20)22-13/h1-6,18H,(H,17,19,20)/b13-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189753
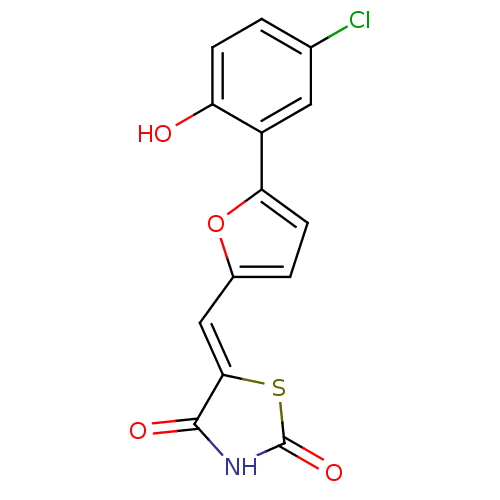
(5-[5-(2-hydroxy-5-chlorophenyl)furan-2-ylmethylene...)Show InChI InChI=1S/C14H8ClNO4S/c15-7-1-3-10(17)9(5-7)11-4-2-8(20-11)6-12-13(18)16-14(19)21-12/h1-6,17H,(H,16,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189742
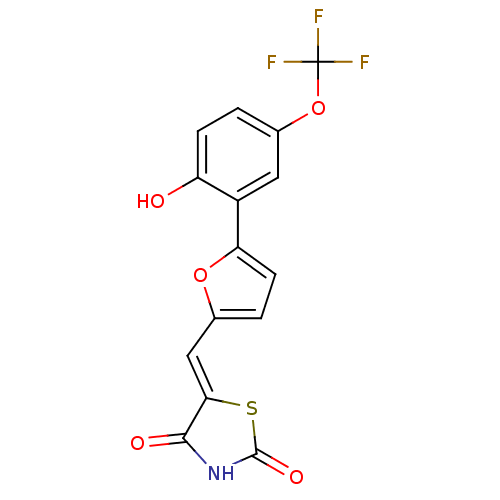
(5-[5-(2-hydroxy-5-trifluoromethoxyphenyl)furan-2-y...)Show SMILES Oc1ccc(OC(F)(F)F)cc1-c1ccc(\C=C2/SC(=O)NC2=O)o1 Show InChI InChI=1S/C15H8F3NO5S/c16-15(17,18)24-8-1-3-10(20)9(5-8)11-4-2-7(23-11)6-12-13(21)19-14(22)25-12/h1-6,20H,(H,19,21,22)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189764
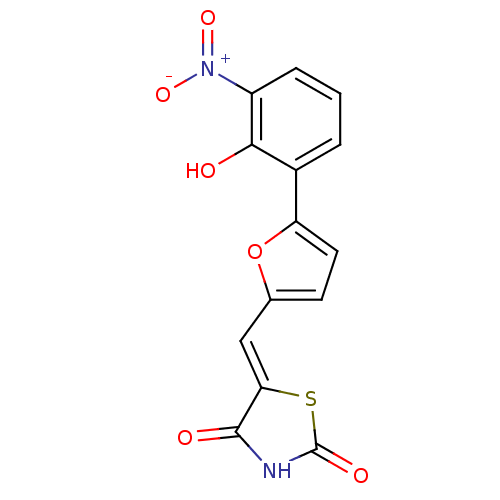
(5-[5-(2-hydroxy-3-nitrophenyl)furan-2-ylmethylene]...)Show SMILES Oc1c(cccc1[N+]([O-])=O)-c1ccc(\C=C2/SC(=O)NC2=O)o1 Show InChI InChI=1S/C14H8N2O6S/c17-12-8(2-1-3-9(12)16(20)21)10-5-4-7(22-10)6-11-13(18)15-14(19)23-11/h1-6,17H,(H,15,18,19)/b11-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25059
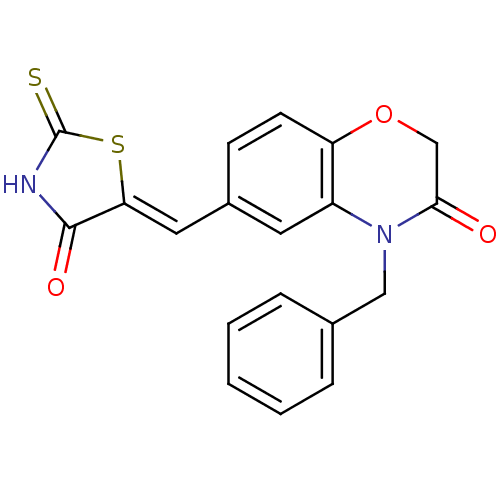
(4-benzyl-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazo...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccccc3)c2c1 Show InChI InChI=1S/C19H14N2O3S2/c22-17-11-24-15-7-6-13(9-16-18(23)20-19(25)26-16)8-14(15)21(17)10-12-4-2-1-3-5-12/h1-9H,10-11H2,(H,20,23,25)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25067
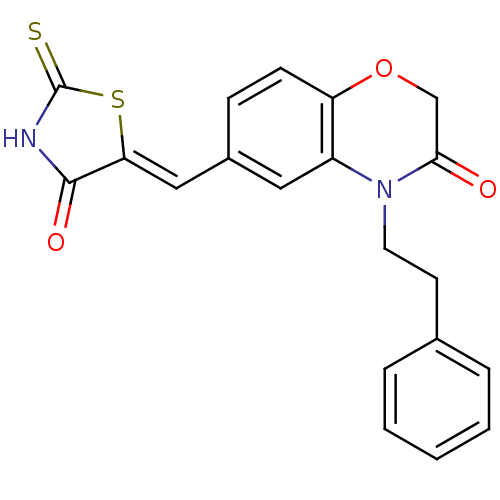
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(CCc3ccccc3)c2c1 Show InChI InChI=1S/C20H16N2O3S2/c23-18-12-25-16-7-6-14(11-17-19(24)21-20(26)27-17)10-15(16)22(18)9-8-13-4-2-1-3-5-13/h1-7,10-11H,8-9,12H2,(H,21,24,26)/b17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189746
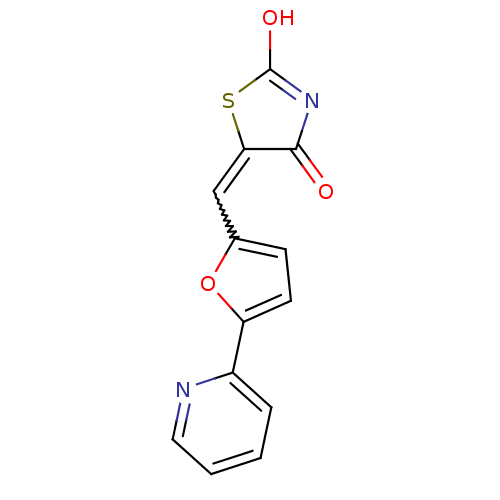
(5-(5-pyridin-2-ylfuran-2-ylmethylene)thiazolidine-...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccccn1 |w:7.8,t:1| Show InChI InChI=1S/C13H8N2O3S/c16-12-11(19-13(17)15-12)7-8-4-5-10(18-8)9-3-1-2-6-14-9/h1-7H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25064
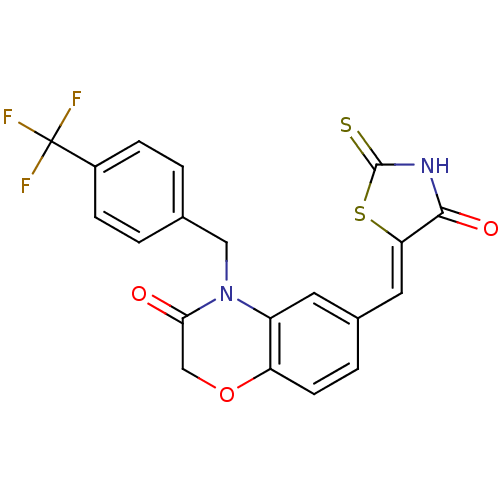
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H13F3N2O3S2/c21-20(22,23)13-4-1-11(2-5-13)9-25-14-7-12(3-6-15(14)28-10-17(25)26)8-16-18(27)24-19(29)30-16/h1-8H,9-10H2,(H,24,27,29)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25060
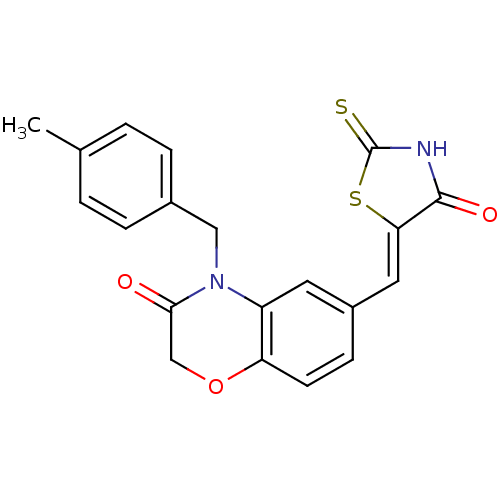
(4-[(4-methylphenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H16N2O3S2/c1-12-2-4-13(5-3-12)10-22-15-8-14(6-7-16(15)25-11-18(22)23)9-17-19(24)21-20(26)27-17/h2-9H,10-11H2,1H3,(H,21,24,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25076
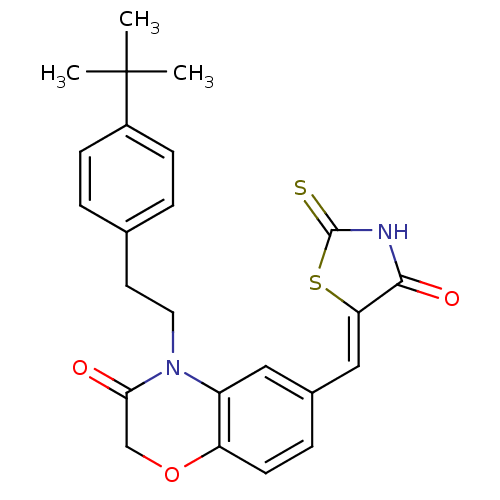
(4-[2-(4-tert-butylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES CC(C)(C)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C24H24N2O3S2/c1-24(2,3)17-7-4-15(5-8-17)10-11-26-18-12-16(6-9-19(18)29-14-21(26)27)13-20-22(28)25-23(30)31-20/h4-9,12-13H,10-11,14H2,1-3H3,(H,25,28,30)/b20-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25063
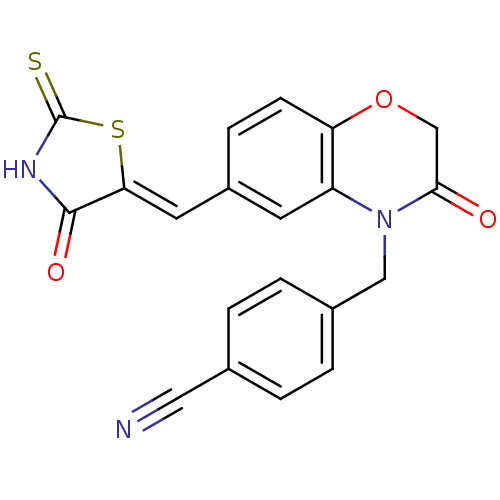
(4-[(3-oxo-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiaz...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccc(cc3)C#N)c2c1 Show InChI InChI=1S/C20H13N3O3S2/c21-9-12-1-3-13(4-2-12)10-23-15-7-14(5-6-16(15)26-11-18(23)24)8-17-19(25)22-20(27)28-17/h1-8H,10-11H2,(H,22,25,27)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25065
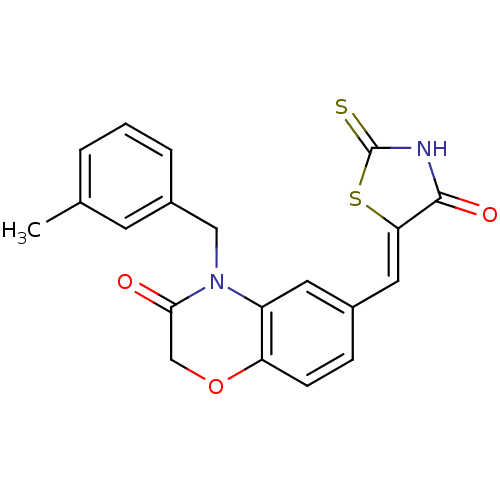
(4-[(3-methylphenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Cc1cccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H16N2O3S2/c1-12-3-2-4-14(7-12)10-22-15-8-13(5-6-16(15)25-11-18(22)23)9-17-19(24)21-20(26)27-17/h2-9H,10-11H2,1H3,(H,21,24,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189766
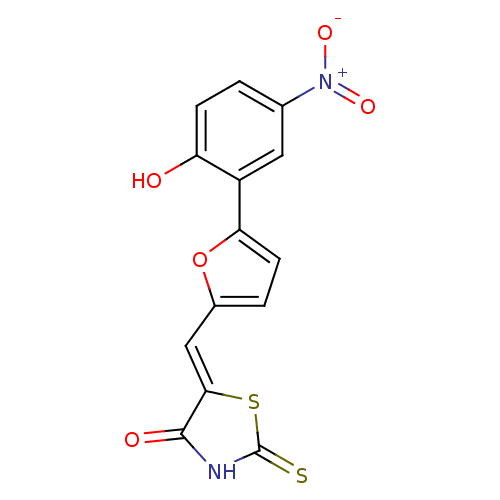
(5-((5-(2-hydroxy-5-nitrophenyl)furan-2-yl)methylen...)Show SMILES Oc1ccc(cc1-c1ccc(\C=C2/SC(=S)NC2=O)o1)[N+]([O-])=O Show InChI InChI=1S/C14H8N2O5S2/c17-10-3-1-7(16(19)20)5-9(10)11-4-2-8(21-11)6-12-13(18)15-14(22)23-12/h1-6,17H,(H,15,18,22)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189756
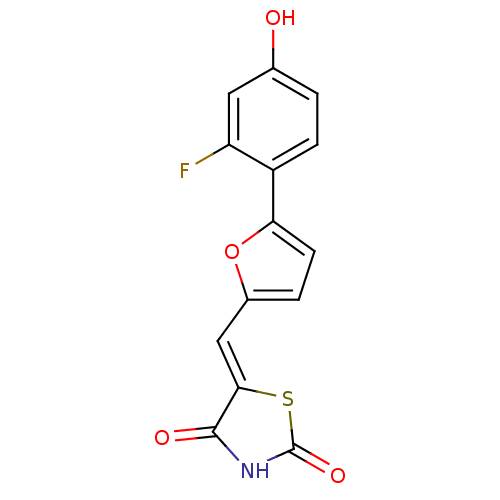
(5-[5-(2-fluoro-4-hydroxyphenyl)furan-2-ylmethylene...)Show InChI InChI=1S/C14H8FNO4S/c15-10-5-7(17)1-3-9(10)11-4-2-8(20-11)6-12-13(18)16-14(19)21-12/h1-6,17H,(H,16,18,19)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189755
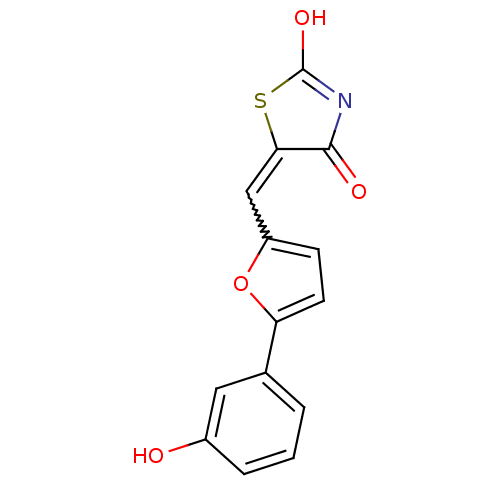
(5-[5-(3-hydroxyphenyl)furan-2-ylmethylene]thiazoli...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1cccc(O)c1 |w:7.8,t:1| Show InChI InChI=1S/C14H9NO4S/c16-9-3-1-2-8(6-9)11-5-4-10(19-11)7-12-13(17)15-14(18)20-12/h1-7,16H,(H,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25058
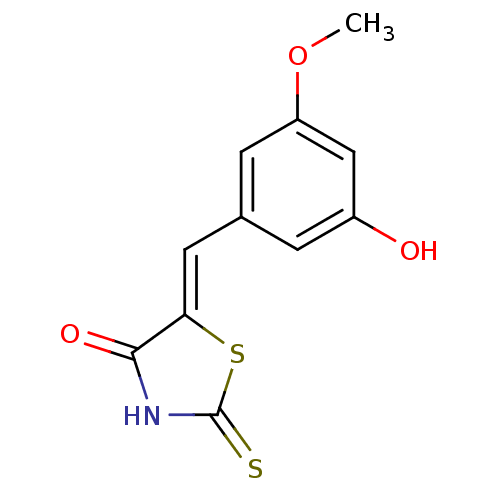
((5Z)-5-[(3-hydroxy-5-methoxyphenyl)methylidene]-2-...)Show InChI InChI=1S/C11H9NO3S2/c1-15-8-3-6(2-7(13)5-8)4-9-10(14)12-11(16)17-9/h2-5,13H,1H3,(H,12,14,16)/b9-4- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189747
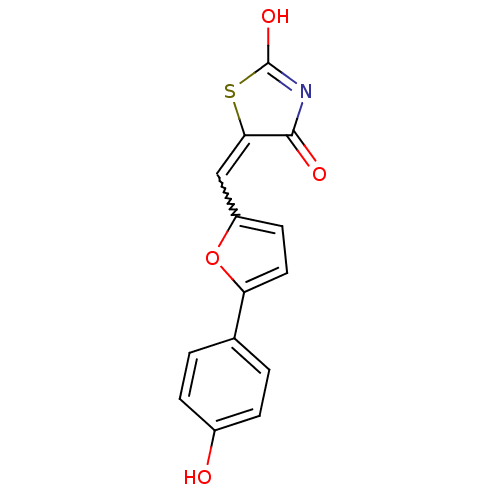
(5-[5-(4-hydroxyphenyl)furan-2-ylmethylene]thiazoli...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccc(O)cc1 |w:7.8,t:1| Show InChI InChI=1S/C14H9NO4S/c16-9-3-1-8(2-4-9)11-6-5-10(19-11)7-12-13(17)15-14(18)20-12/h1-7,16H,(H,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human GST-CDK1/cyclin B1 expressed in baculovirus using [gamma-33P]ATP after 45 mins by liquid scintillation counting |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189758
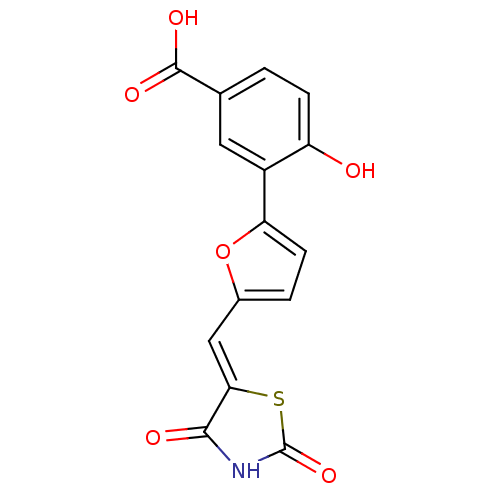
(3-[5-(2,4-dioxothiazolidin-5-ylidenemethyl)furan-2...)Show SMILES OC(=O)c1ccc(O)c(c1)-c1ccc(\C=C2/SC(=O)NC2=O)o1 Show InChI InChI=1S/C15H9NO6S/c17-10-3-1-7(14(19)20)5-9(10)11-4-2-8(22-11)6-12-13(18)16-15(21)23-12/h1-6,17H,(H,19,20)(H,16,18,21)/b12-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50326169
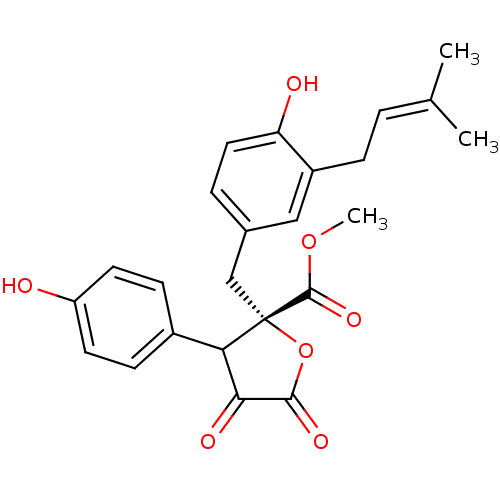
((S)-methyl 4-hydroxy-2-(4-hydroxy-3-(3-methylbut-2...)Show SMILES [#6]-[#8]-[#6](=O)[C@@]1([#6]-c2ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2)[#8]-[#6](=O)-[#6](=O)-[#6]1-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C24H24O7/c1-14(2)4-6-17-12-15(5-11-19(17)26)13-24(23(29)30-3)20(21(27)22(28)31-24)16-7-9-18(25)10-8-16/h4-5,7-12,20,25-26H,6,13H2,1-3H3/t20?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human GST-CDK1/cyclin B1 expressed in baculovirus using [gamma-33P]ATP after 45 mins by liquid scintillation counting |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50326170

(2-((3-(7-chloroquinolin-4-ylamino)propyl)(ethyl)am...)Show InChI InChI=1S/C16H22ClN3O/c1-2-20(10-11-21)9-3-7-18-15-6-8-19-16-12-13(17)4-5-14(15)16/h4-6,8,12,21H,2-3,7,9-11H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189768
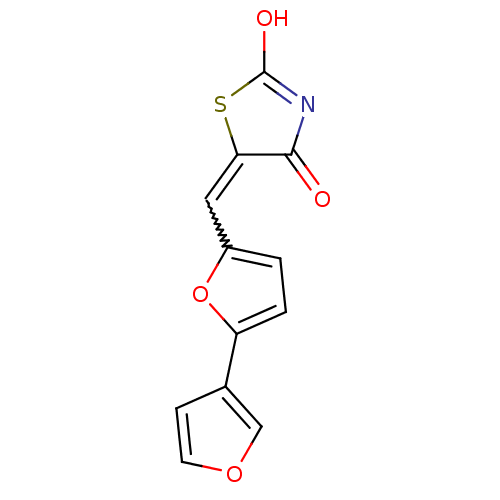
(5-[2,3']bifuranyl-5-ylmethylenethiazolidine-2,4-di...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccoc1 |w:7.8,t:1| Show InChI InChI=1S/C12H7NO4S/c14-11-10(18-12(15)13-11)5-8-1-2-9(17-8)7-3-4-16-6-7/h1-6H,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50326171
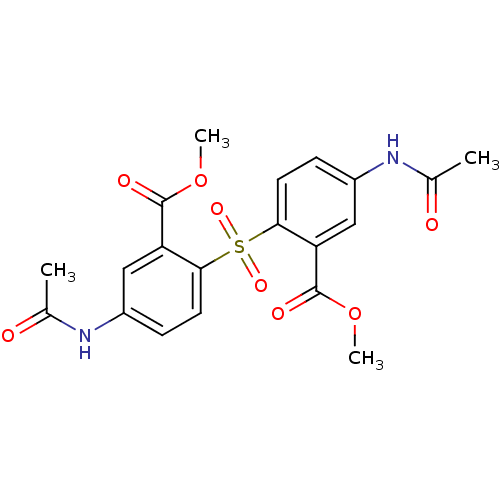
(CHEMBL1243004 | dimethyl 6,6'-sulfonylbis(3-acetam...)Show SMILES COC(=O)c1cc(NC(C)=O)ccc1S(=O)(=O)c1ccc(NC(C)=O)cc1C(=O)OC Show InChI InChI=1S/C20H20N2O8S/c1-11(23)21-13-5-7-17(15(9-13)19(25)29-3)31(27,28)18-8-6-14(22-12(2)24)10-16(18)20(26)30-4/h5-10H,1-4H3,(H,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Eur J Med Chem 45: 4316-30 (2010)
Article DOI: 10.1016/j.ejmech.2010.06.034
BindingDB Entry DOI: 10.7270/Q2W66KZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189763
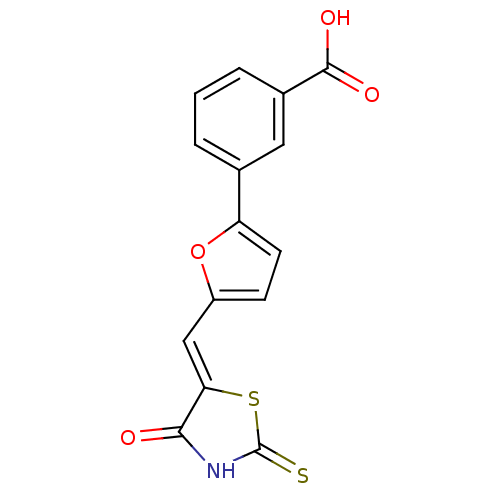
(3-(5-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)...)Show SMILES OC(=O)c1cccc(c1)-c1ccc(\C=C2/SC(=S)NC2=O)o1 Show InChI InChI=1S/C15H9NO4S2/c17-13-12(22-15(21)16-13)7-10-4-5-11(20-10)8-2-1-3-9(6-8)14(18)19/h1-7H,(H,18,19)(H,16,17,21)/b12-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189765
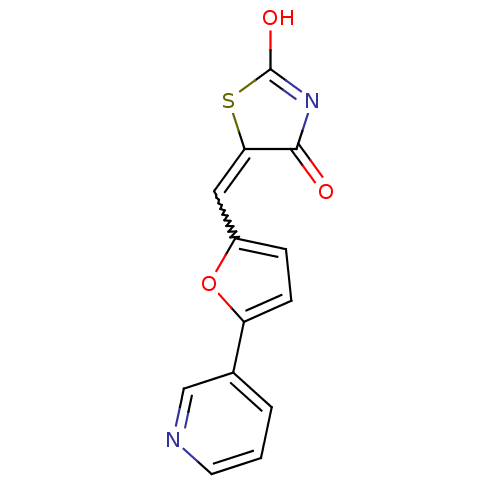
(5-(5-pyridin-3-ylfuran-2-ylmethylene)thiazolidine-...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1cccnc1 |w:7.8,t:1| Show InChI InChI=1S/C13H8N2O3S/c16-12-11(19-13(17)15-12)6-9-3-4-10(18-9)8-2-1-5-14-7-8/h1-7H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data