 Found 216 hits with Last Name = 'alberts' and Initial = 'aw'
Found 216 hits with Last Name = 'alberts' and Initial = 'aw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-arrestin-1
(RABBIT) | BDBM82381
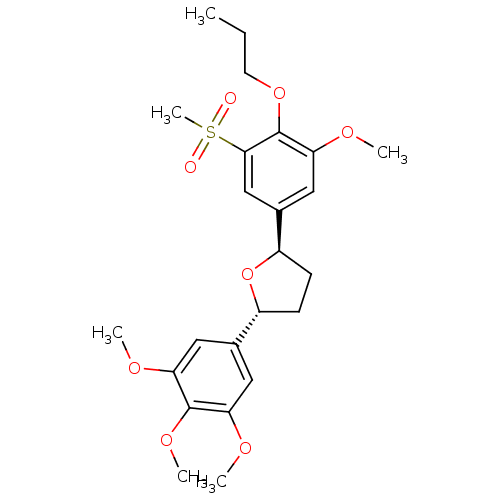
(L-659,989, (-))Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@H]1CC[C@@H](O1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50280339

((S)-2-[4-(2-Benzenesulfonyl-ethoxy)-3-methoxy-5-(p...)Show SMILES CCCS(=O)(=O)c1cc(cc(OC)c1OCCS(=O)(=O)c1ccccc1)C1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H38O10S2/c1-6-15-43(34,35)29-20-22(19-28(38-4)31(29)40-14-16-42(32,33)23-10-8-7-9-11-23)25-13-12-24(41-25)21-17-26(36-2)30(39-5)27(18-21)37-3/h7-11,17-20,24-25H,6,12-16H2,1-5H3/t24-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of binding of [3H]-C18 PAF to human platelet membrane Platelet activating factor receptor |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Beta-arrestin-1
(RABBIT) | BDBM50002829
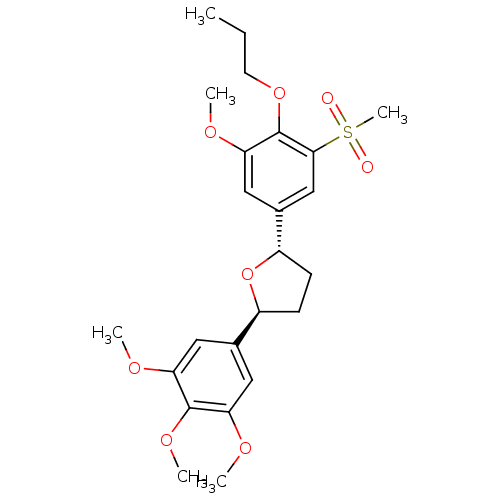
((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002827

(1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...)Show SMILES CCCOc1c(OCCCO)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H38O10S/c1-6-11-37-28-25(36-12-7-10-29)15-20(16-26(28)39(31,32)17-18(2)30)22-9-8-21(38-22)19-13-23(33-3)27(35-5)24(14-19)34-4/h13-16,21-22,29H,6-12,17H2,1-5H3/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002828
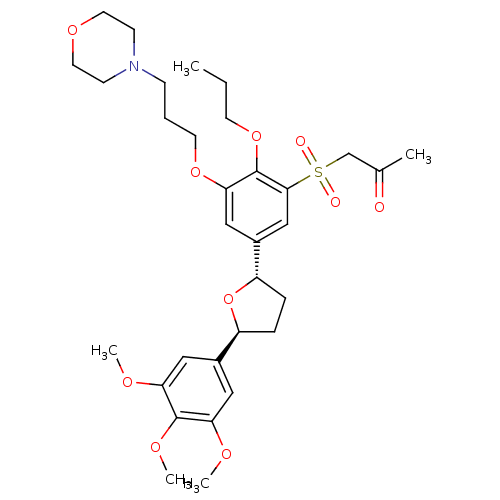
(1-{3-(3-Morpholin-4-yl-propoxy)-2-propoxy-5-[(2S,5...)Show SMILES CCCOc1c(OCCCN2CCOCC2)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C32H45NO10S/c1-6-13-42-32-29(41-14-7-10-33-11-15-40-16-12-33)19-24(20-30(32)44(35,36)21-22(2)34)26-9-8-25(43-26)23-17-27(37-3)31(39-5)28(18-23)38-4/h17-20,25-26H,6-16,21H2,1-5H3/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002824
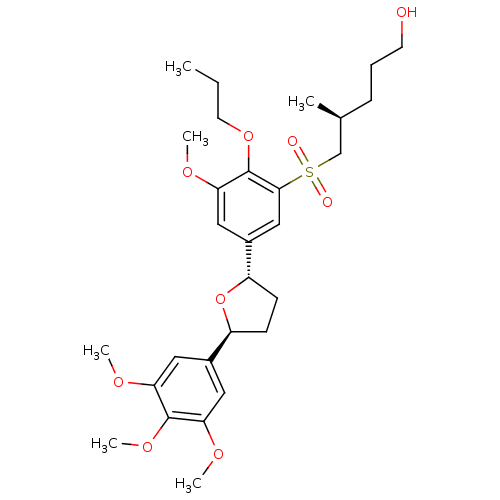
((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002827

(1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...)Show SMILES CCCOc1c(OCCCO)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H38O10S/c1-6-11-37-28-25(36-12-7-10-29)15-20(16-26(28)39(31,32)17-18(2)30)22-9-8-21(38-22)19-13-23(33-3)27(35-5)24(14-19)34-4/h13-16,21-22,29H,6-12,17H2,1-5H3/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002826
(1-{3-(3-Imidazol-1-yl-propoxy)-2-propoxy-5-[(2S,5S...)Show SMILES CCCOc1c(OCCCn2ccnc2)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H40N2O9S/c1-6-13-41-31-28(40-14-7-11-33-12-10-32-20-33)17-23(18-29(31)43(35,36)19-21(2)34)25-9-8-24(42-25)22-15-26(37-3)30(39-5)27(16-22)38-4/h10,12,15-18,20,24-25H,6-9,11,13-14,19H2,1-5H3/t24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823
(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002825

((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002824

((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002825

((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)C[C@H](C)CCCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C29H42O9S/c1-7-13-37-29-26(35-5)16-21(17-27(29)39(31,32)18-19(2)9-8-12-30)23-11-10-22(38-23)20-14-24(33-3)28(36-6)25(15-20)34-4/h14-17,19,22-23,30H,7-13,18H2,1-6H3/t19-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM82381

(L-659,989, (-))Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@H]1CC[C@@H](O1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-C18 PAF binding to human platelet membrane Platelet activating factor receptor was determined |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to PMN membrane receptors |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81972
((S)-(+)-ALPHA-METHYL-1H-IMIDAZOLE-4-ETHANAMINE DIH...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Beta-arrestin-1
(RABBIT) | BDBM50002829

((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81972

((S)-(+)-ALPHA-METHYL-1H-IMIDAZOLE-4-ETHANAMINE DIH...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-C18 PAF binding to human platelet membrane Platelet activating factor receptor was determined |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002829

((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50000714
(3-[4-(2-Chloro-phenyl)-9-methyl-6H-1-thia-5,7,8,9a...)Show SMILES Cc1nnc2CN=C(c3cc(CCC(=O)N4CCOCC4)sc3-n12)c1ccccc1Cl |c:6| Show InChI InChI=1S/C22H22ClN5O2S/c1-14-25-26-19-13-24-21(16-4-2-3-5-18(16)23)17-12-15(31-22(17)28(14)19)6-7-20(29)27-8-10-30-11-9-27/h2-5,12H,6-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 79.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50366241
(CHEMBL297624 | L-652731)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CC[C@@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H28O7/c1-23-17-9-13(10-18(24-2)21(17)27-5)15-7-8-16(29-15)14-11-19(25-3)22(28-6)20(12-14)26-4/h9-12,15-16H,7-8H2,1-6H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50366241

(CHEMBL297624 | L-652731)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CC[C@@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H28O7/c1-23-17-9-13(10-18(24-2)21(17)27-5)15-7-8-16(29-15)14-11-19(25-3)22(28-6)20(12-14)26-4/h9-12,15-16H,7-8H2,1-6H3/t15-,16-/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration required to inhibit PAF binding to rabbit platelet membrane |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002822

((-)-trans 42s,5S)-2-[3-[(2-Oxopropyl)sulfony1]-4-n...)Show SMILES CCCOc1c(OCCCOP(O)([O-])=O)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H39O13PS/c1-6-10-39-28-25(38-11-7-12-40-42(30,31)32)15-20(16-26(28)43(33,34)17-18(2)29)22-9-8-21(41-22)19-13-23(35-3)27(37-5)24(14-19)36-4/h13-16,21-22H,6-12,17H2,1-5H3,(H2,30,31,32)/p-1/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823

(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human platelet |
Bioorg Med Chem Lett 1: 327-332 (1991)
Article DOI: 10.1016/S0960-894X(01)80818-0
BindingDB Entry DOI: 10.7270/Q2TM7BKP |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002829

((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002822

((-)-trans 42s,5S)-2-[3-[(2-Oxopropyl)sulfony1]-4-n...)Show SMILES CCCOc1c(OCCCOP(O)([O-])=O)cc(cc1S(=O)(=O)CC(C)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H39O13PS/c1-6-10-39-28-25(38-11-7-12-40-42(30,31)32)15-20(16-26(28)43(33,34)17-18(2)29)22-9-8-21(41-22)19-13-23(35-3)27(37-5)24(14-19)36-4/h13-16,21-22H,6-12,17H2,1-5H3,(H2,30,31,32)/p-1/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Beta-arrestin-1
(RABBIT) | BDBM50002829

((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002830
((2R,3R,4R,5S)-2,5-Bis-(3,4-dimethoxy-phenyl)-3,4-d...)Show SMILES COc1ccc(cc1OC)[C@H]1O[C@H]([C@H](C)[C@H]1C)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C22H28O5/c1-13-14(2)22(16-8-10-18(24-4)20(12-16)26-6)27-21(13)15-7-9-17(23-3)19(11-15)25-5/h7-14,21-22H,1-6H3/t13-,14-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 35: 3474-82 (1992)
BindingDB Entry DOI: 10.7270/Q2445N3B |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002829

((2S,5S)-2-(3-Methanesulfonyl-5-methoxy-4-propoxy-p...)Show SMILES CCCOc1c(OC)cc(cc1S(C)(=O)=O)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H32O8S/c1-7-10-31-24-21(29-4)13-16(14-22(24)33(6,25)26)18-9-8-17(32-18)15-11-19(27-2)23(30-5)20(12-15)28-3/h11-14,17-18H,7-10H2,1-6H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 246: 534-41 (1988)
BindingDB Entry DOI: 10.7270/Q2CJ8BZ8 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50003240

(2,2-Dimethyl-butyric acid 8-[2-(4-hydroxy-6-oxo-te...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19?,20-,21-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50280339

((S)-2-[4-(2-Benzenesulfonyl-ethoxy)-3-methoxy-5-(p...)Show SMILES CCCS(=O)(=O)c1cc(cc(OC)c1OCCS(=O)(=O)c1ccccc1)C1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C31H38O10S2/c1-6-15-43(34,35)29-20-22(19-28(38-4)31(29)40-14-16-42(32,33)23-10-8-7-9-11-23)25-13-12-24(41-25)21-17-26(36-2)30(39-5)27(18-21)37-3/h7-11,17-20,24-25H,6,12-16H2,1-5H3/t24-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity of binding of [3H]-C18 PAF to human platelet membrane Platelet activating factor receptor |
Bioorg Med Chem Lett 2: 181-184 (1992)
Article DOI: 10.1016/S0960-894X(01)80446-7
BindingDB Entry DOI: 10.7270/Q24X588Z |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50226270

(CHEMBL3349956)Show SMILES CCC(CC)(CC)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:16,t:14| Show InChI InChI=1S/C27H42O5/c1-6-27(7-2,8-3)26(30)32-23-14-17(4)13-19-10-9-18(5)22(25(19)23)12-11-21-15-20(28)16-24(29)31-21/h9-10,13,17-18,20-23,25,28H,6-8,11-12,14-16H2,1-5H3/t17-,18-,20+,21+,22-,23-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50226272
(CHEMBL3349944)Show SMILES CCC(C)(CC)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:15,t:13| Show InChI InChI=1S/C26H40O5/c1-6-26(5,7-2)25(29)31-22-13-16(3)12-18-9-8-17(4)21(24(18)22)11-10-20-14-19(27)15-23(28)30-20/h8-9,12,16-17,19-22,24,27H,6-7,10-11,13-15H2,1-5H3/t16-,17-,19+,20+,21-,22-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50226271

(CHEMBL3349955)Show SMILES CCCC(CC)(CC)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:17,t:15| Show InChI InChI=1S/C28H44O5/c1-6-13-28(7-2,8-3)27(31)33-24-15-18(4)14-20-10-9-19(5)23(26(20)24)12-11-22-16-21(29)17-25(30)32-22/h9-10,14,18-19,21-24,26,29H,6-8,11-13,15-17H2,1-5H3/t18-,19-,21+,22+,23-,24-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50003235
(2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...)Show SMILES [H][C@](C)(CC)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:14,t:12| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024628

(2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...)Show SMILES [H][C@@](C)(CC)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:14,t:12| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15+,16-,18+,19?,20-,21-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024637
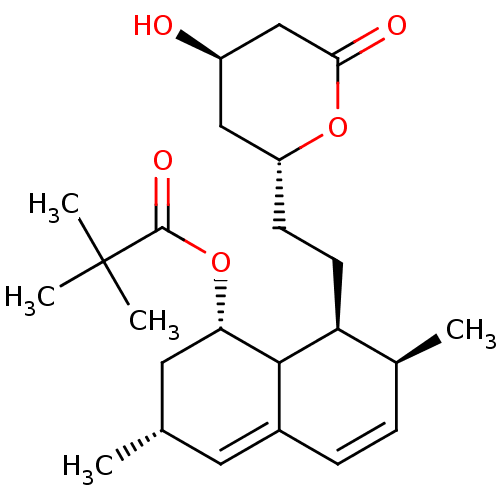
(2,2-Diethyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tet...)Show SMILES C[C@@H]1C[C@H](OC(=O)C(C)(C)C)C2[C@@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H](C)C=CC2=C1 |c:26,29| Show InChI InChI=1S/C27H42O5/c1-6-27(7-2,8-3)26(30)32-23-14-17(4)13-19-10-9-18(5)22(25(19)23)12-11-21-15-20(28)16-24(29)31-21/h9-10,13,17-18,20-23,25,28H,6-8,11-12,14-16H2,1-5H3/t17-,18-,20+,21?,22-,23-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50009407

(But-3-enoic acid 8-[2-(4-hydroxy-6-oxo-tetrahydro-...)Show SMILES C[C@@H]1C[C@H](OC(=O)CC=C)[C@@H]2[C@@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H](C)C=CC2=C1 |c:25,28| Show InChI InChI=1S/C23H32O5/c1-4-5-21(25)28-20-11-14(2)10-16-7-6-15(3)19(23(16)20)9-8-18-12-17(24)13-22(26)27-18/h4,6-7,10,14-15,17-20,23-24H,1,5,8-9,11-13H2,2-3H3/t14-,15-,17+,18?,19-,20-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against rat liver HMG-CoA reductase enzyme |
J Med Chem 34: 2474-7 (1991)
BindingDB Entry DOI: 10.7270/Q29S1Q0D |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024642
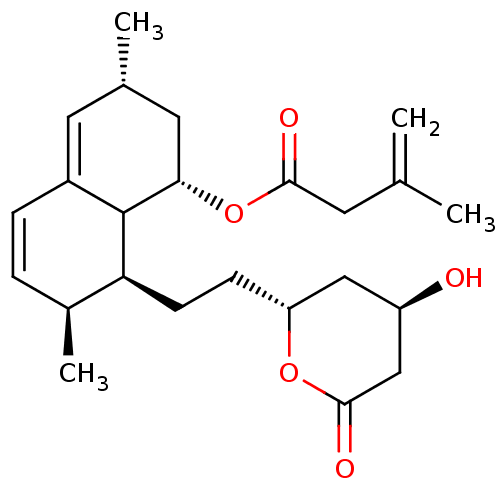
(3-Methyl-but-3-enoic acid 8-[2-(4-hydroxy-6-oxo-te...)Show SMILES C[C@@H]1C[C@H](OC(=O)CC(C)=C)C2[C@@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H](C)C=CC2=C1 |c:26,29| Show InChI InChI=1S/C24H34O5/c1-14(2)9-22(26)29-21-11-15(3)10-17-6-5-16(4)20(24(17)21)8-7-19-12-18(25)13-23(27)28-19/h5-6,10,15-16,18-21,24-25H,1,7-9,11-13H2,2-4H3/t15-,16-,18+,19?,20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024630

(4,4,4-Trifluoro-3-methyl-butyric acid 8-[2-(4-hydr...)Show SMILES CC(CC(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12)C(F)(F)F |c:12,t:10| Show InChI InChI=1S/C24H33F3O5/c1-13-8-16-5-4-14(2)19(7-6-18-11-17(28)12-22(30)31-18)23(16)20(9-13)32-21(29)10-15(3)24(25,26)27/h4-5,8,13-15,17-20,23,28H,6-7,9-12H2,1-3H3/t13-,14-,15?,17+,18?,19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024638
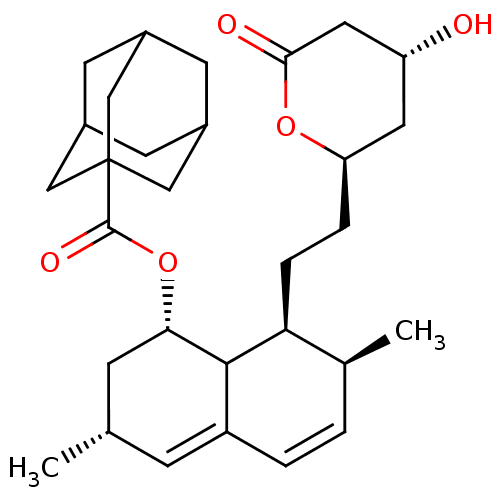
(Adamantane-1-carboxylic acid 8-[2-(4-hydroxy-6-oxo...)Show SMILES C[C@@H]1C[C@H](OC(=O)C23CC4CC(CC(C4)C2)C3)C2[C@@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H](C)C=CC2=C1 |c:35,38,THB:12:11:8:15.13.14| Show InChI InChI=1S/C30H42O5/c1-17-7-22-4-3-18(2)25(6-5-24-12-23(31)13-27(32)34-24)28(22)26(8-17)35-29(33)30-14-19-9-20(15-30)11-21(10-19)16-30/h3-4,7,17-21,23-26,28,31H,5-6,8-16H2,1-2H3/t17-,18-,19?,20?,21?,23+,24?,25-,26-,28?,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50009411
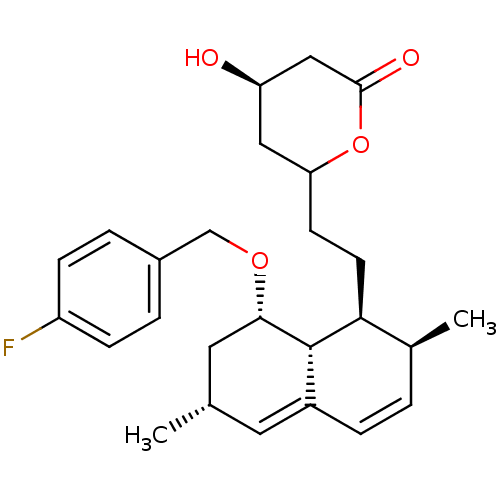
(6-{2-[8-(4-Fluoro-benzyloxy)-2,6-dimethyl-1,2,6,7,...)Show SMILES C[C@@H]1C[C@H](OCc2ccc(F)cc2)[C@@H]2[C@@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H](C)C=CC2=C1 |c:29,32| Show InChI InChI=1S/C26H33FO4/c1-16-11-19-6-3-17(2)23(10-9-22-13-21(28)14-25(29)31-22)26(19)24(12-16)30-15-18-4-7-20(27)8-5-18/h3-8,11,16-17,21-24,26,28H,9-10,12-15H2,1-2H3/t16-,17-,21+,22?,23-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against rat liver HMG-CoA reductase enzyme |
J Med Chem 34: 2474-7 (1991)
BindingDB Entry DOI: 10.7270/Q29S1Q0D |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024972
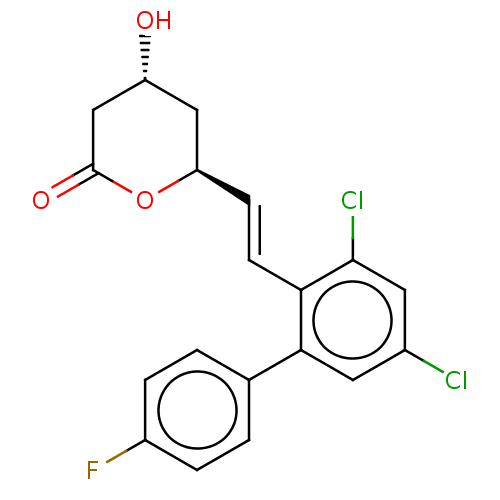
((+) 6-[2-(3,5-Dichloro-4''-fluoro-biphenyl-2-yl)-v...)Show SMILES O[C@@H]1C[C@H](OC(=O)C1)\C=C\c1c(Cl)cc(Cl)cc1-c1ccc(F)cc1 Show InChI InChI=1S/C19H15Cl2FO3/c20-12-7-17(11-1-3-13(22)4-2-11)16(18(21)8-12)6-5-15-9-14(23)10-19(24)25-15/h1-8,14-15,23H,9-10H2/b6-5+/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized partially purified rat liver HMG-CoA reductase |
J Med Chem 29: 170-81 (1986)
BindingDB Entry DOI: 10.7270/Q2RV0MQX |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024972

((+) 6-[2-(3,5-Dichloro-4''-fluoro-biphenyl-2-yl)-v...)Show SMILES O[C@@H]1C[C@H](OC(=O)C1)\C=C\c1c(Cl)cc(Cl)cc1-c1ccc(F)cc1 Show InChI InChI=1S/C19H15Cl2FO3/c20-12-7-17(11-1-3-13(22)4-2-11)16(18(21)8-12)6-5-15-9-14(23)10-19(24)25-15/h1-8,14-15,23H,9-10H2/b6-5+/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized partially purified rat liver HMG-CoA reductase |
J Med Chem 29: 170-81 (1986)
BindingDB Entry DOI: 10.7270/Q2RV0MQX |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50009426

(2,2-Dimethyl-butyric acid 4-benzylcarbamoyloxy-8-[...)Show SMILES [H][C@@]12CC[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C1[C@H](C[C@@H](C)[C@H]2OC(=O)NCc1ccccc1)OC(=O)C(C)(C)CC Show InChI InChI=1S/C33H49NO7/c1-6-33(4,5)31(37)40-27-16-21(3)30(41-32(38)34-19-22-10-8-7-9-11-22)26-14-12-20(2)25(29(26)27)15-13-24-17-23(35)18-28(36)39-24/h7-11,20-21,23-27,29-30,35H,6,12-19H2,1-5H3,(H,34,38)/t20-,21+,23+,24?,25-,26+,27-,29?,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit solubilized, partially purified, rat liver HMG-CoA reductase |
J Med Chem 34: 2489-95 (1991)
BindingDB Entry DOI: 10.7270/Q2610Z80 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50024629

(3-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...)Show SMILES CC(C)CC(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-14(2)9-22(26)29-21-11-15(3)10-17-6-5-16(4)20(24(17)21)8-7-19-12-18(25)13-23(27)28-19/h5-6,10,14-16,18-21,24-25H,7-9,11-13H2,1-4H3/t15-,16-,18+,19?,20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 849-52 (1986)
BindingDB Entry DOI: 10.7270/Q2BZ652M |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50406550

(CHEMBL2114188 | CHEMBL3349570)Show SMILES [H][C@@]12CC[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)C1[C@H](C[C@](C)(CSc1ccccc1)O2)OC(=O)C(C)(C)CC Show InChI InChI=1S/C31H46O6S/c1-6-30(3,4)29(34)36-26-18-31(5,19-38-23-10-8-7-9-11-23)37-25-15-12-20(2)24(28(25)26)14-13-22-16-21(32)17-27(33)35-22/h7-11,20-22,24-26,28,32H,6,12-19H2,1-5H3/t20-,21+,22?,24-,25+,26-,28-,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit solubilized, partially purified, rat liver HMG-CoA reductase |
J Med Chem 34: 2489-95 (1991)
BindingDB Entry DOI: 10.7270/Q2610Z80 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data