 Found 196 hits with Last Name = 'alex' and Initial = 'a'
Found 196 hits with Last Name = 'alex' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440172
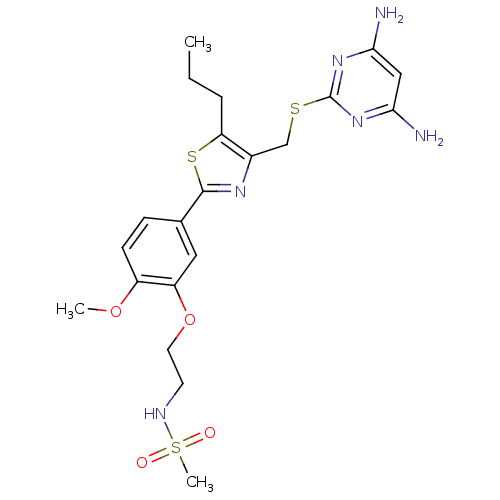
(CHEMBL2426574)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C21H28N6O4S3/c1-4-5-17-14(12-32-21-26-18(22)11-19(23)27-21)25-20(33-17)13-6-7-15(30-2)16(10-13)31-9-8-24-34(3,28)29/h6-7,10-11,24H,4-5,8-9,12H2,1-3H3,(H4,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440176
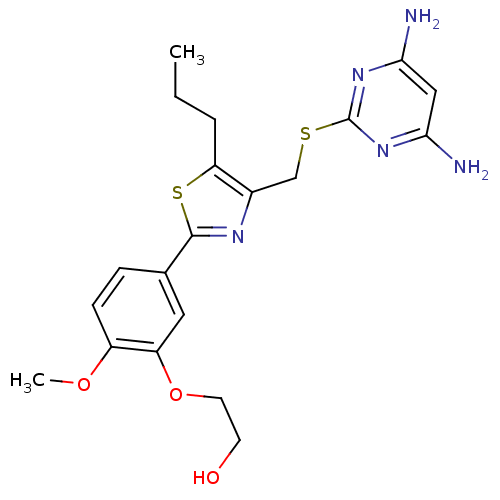
(CHEMBL2426570)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCO)c1 Show InChI InChI=1S/C20H25N5O3S2/c1-3-4-16-13(11-29-20-24-17(21)10-18(22)25-20)23-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-26/h5-6,9-10,26H,3-4,7-8,11H2,1-2H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440172

(CHEMBL2426574)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C21H28N6O4S3/c1-4-5-17-14(12-32-21-26-18(22)11-19(23)27-21)25-20(33-17)13-6-7-15(30-2)16(10-13)31-9-8-24-34(3,28)29/h6-7,10-11,24H,4-5,8-9,12H2,1-3H3,(H4,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173
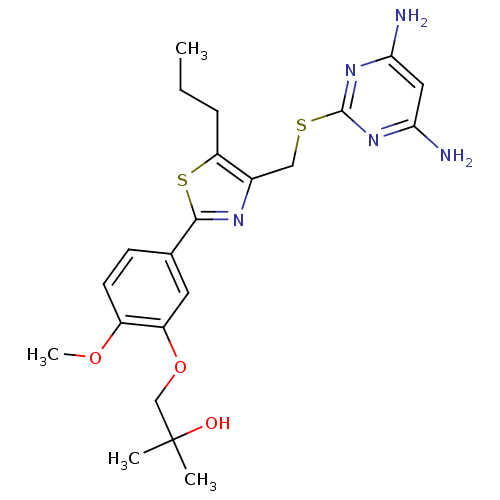
(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173

(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440151
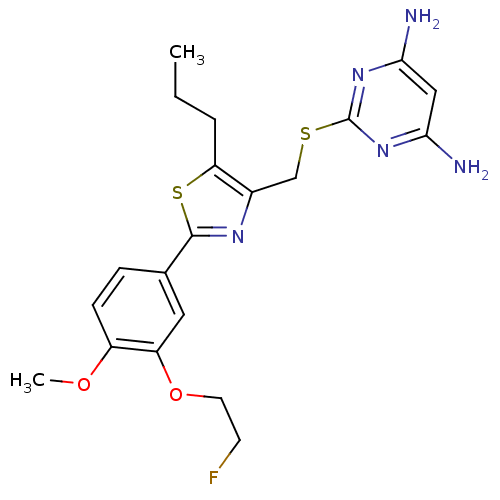
(CHEMBL2426558)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C20H24FN5O2S2/c1-3-4-16-13(11-29-20-25-17(22)10-18(23)26-20)24-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-21/h5-6,9-10H,3-4,7-8,11H2,1-2H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031352
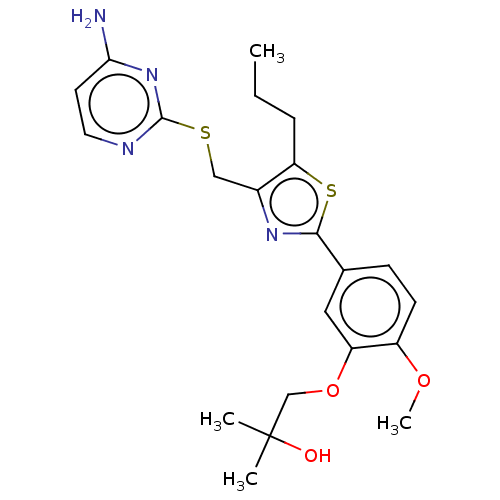
(CHEMBL3358091)Show SMILES CCCc1sc(nc1CSc1nccc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H28N4O3S2/c1-5-6-18-15(12-30-21-24-10-9-19(23)26-21)25-20(31-18)14-7-8-16(28-4)17(11-14)29-13-22(2,3)27/h7-11,27H,5-6,12-13H2,1-4H3,(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440181
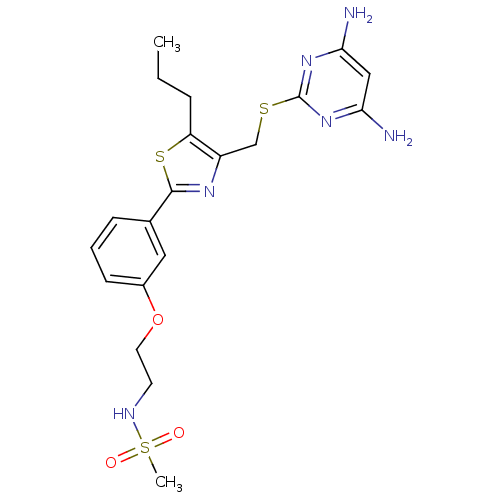
(CHEMBL2426565)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1cccc(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C20H26N6O3S3/c1-3-5-16-15(12-30-20-25-17(21)11-18(22)26-20)24-19(31-16)13-6-4-7-14(10-13)29-9-8-23-32(2,27)28/h4,6-7,10-11,23H,3,5,8-9,12H2,1-2H3,(H4,21,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440179
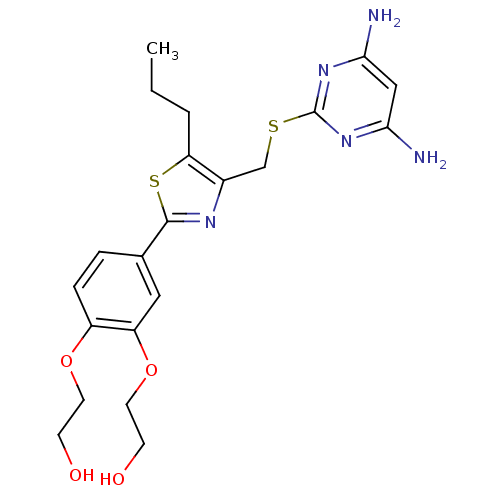
(CHEMBL2426567)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OCCO)c(OCCO)c1 Show InChI InChI=1S/C21H27N5O4S2/c1-2-3-17-14(12-31-21-25-18(22)11-19(23)26-21)24-20(32-17)13-4-5-15(29-8-6-27)16(10-13)30-9-7-28/h4-5,10-11,27-28H,2-3,6-9,12H2,1H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031349
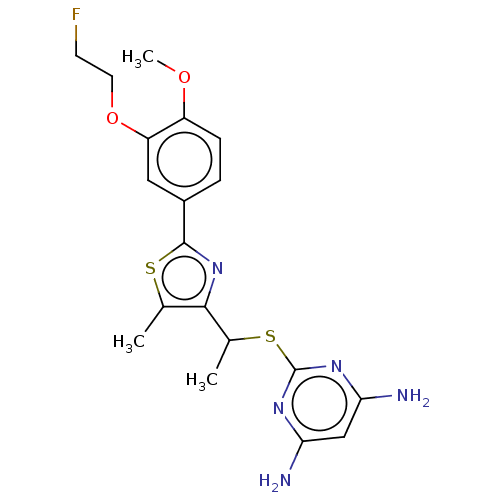
(CHEMBL3358094)Show SMILES COc1ccc(cc1OCCF)-c1nc(C(C)Sc2nc(N)cc(N)n2)c(C)s1 Show InChI InChI=1S/C19H22FN5O2S2/c1-10-17(11(2)29-19-23-15(21)9-16(22)24-19)25-18(28-10)12-4-5-13(26-3)14(8-12)27-7-6-20/h4-5,8-9,11H,6-7H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440178
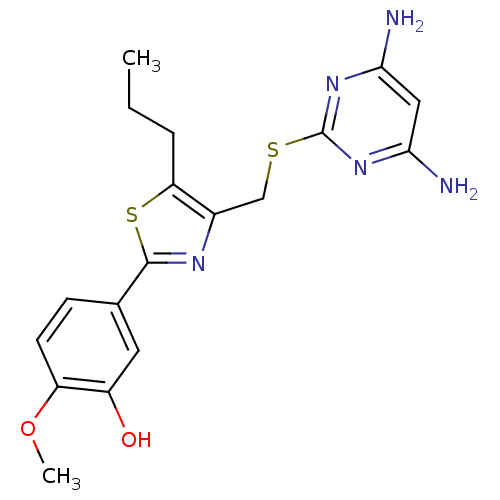
(CHEMBL2426568)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(O)c1 Show InChI InChI=1S/C18H21N5O2S2/c1-3-4-14-11(9-26-18-22-15(19)8-16(20)23-18)21-17(27-14)10-5-6-13(25-2)12(24)7-10/h5-8,24H,3-4,9H2,1-2H3,(H4,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031337
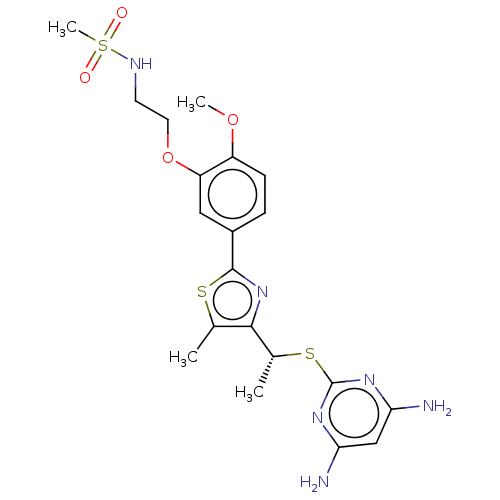
(CHEMBL3358097)Show SMILES COc1ccc(cc1OCCNS(C)(=O)=O)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C20H26N6O4S3/c1-11-18(12(2)32-20-24-16(21)10-17(22)25-20)26-19(31-11)13-5-6-14(29-3)15(9-13)30-8-7-23-33(4,27)28/h5-6,9-10,12,23H,7-8H2,1-4H3,(H4,21,22,24,25)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440140
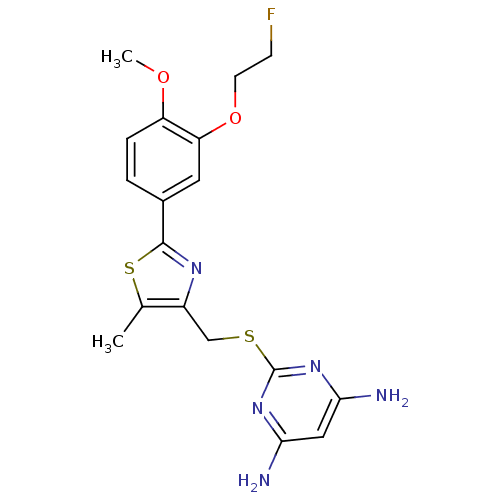
(CHEMBL2426588)Show SMILES COc1ccc(cc1OCCF)-c1nc(CSc2nc(N)cc(N)n2)c(C)s1 Show InChI InChI=1S/C18H20FN5O2S2/c1-10-12(9-27-18-23-15(20)8-16(21)24-18)22-17(28-10)11-3-4-13(25-2)14(7-11)26-6-5-19/h3-4,7-8H,5-6,9H2,1-2H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031339
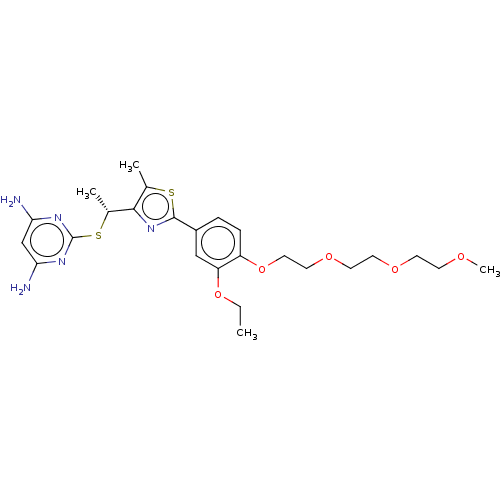
(CHEMBL3358096)Show SMILES CCOc1cc(ccc1OCCOCCOCCOC)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C25H35N5O5S2/c1-5-34-20-14-18(6-7-19(20)35-13-12-33-11-10-32-9-8-31-4)24-30-23(16(2)36-24)17(3)37-25-28-21(26)15-22(27)29-25/h6-7,14-15,17H,5,8-13H2,1-4H3,(H4,26,27,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031350
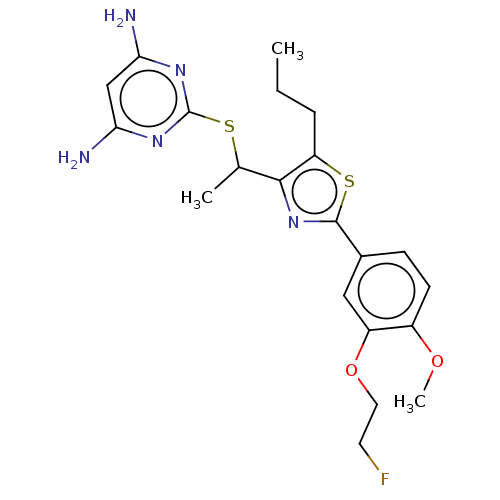
(CHEMBL3358093)Show SMILES CCCc1sc(nc1C(C)Sc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C21H26FN5O2S2/c1-4-5-16-19(12(2)30-21-25-17(23)11-18(24)26-21)27-20(31-16)13-6-7-14(28-3)15(10-13)29-9-8-22/h6-7,10-12H,4-5,8-9H2,1-3H3,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity choline transporter 1
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]hemicholinium-3 from recombinant human choline transporter after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031338
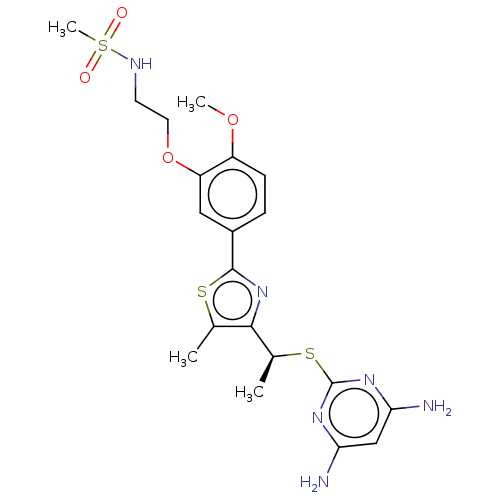
(CHEMBL3358090)Show SMILES COc1ccc(cc1OCCNS(C)(=O)=O)-c1nc([C@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C20H26N6O4S3/c1-11-18(12(2)32-20-24-16(21)10-17(22)25-20)26-19(31-11)13-5-6-14(29-3)15(9-13)30-8-7-23-33(4,27)28/h5-6,9-10,12,23H,7-8H2,1-4H3,(H4,21,22,24,25)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031351
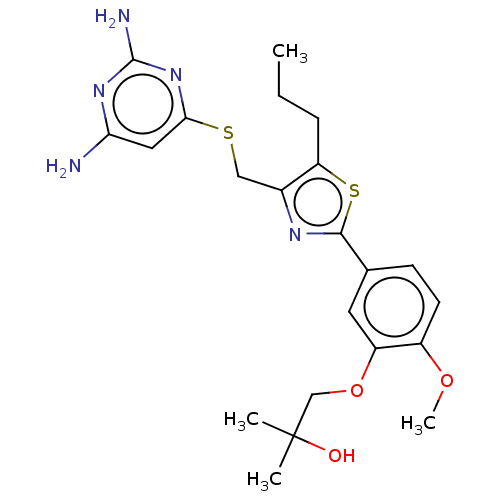
(CHEMBL3358092)Show SMILES CCCc1sc(nc1CSc1cc(N)nc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-19-10-18(23)26-21(24)27-19)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from recombinant human ERG after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from recombinant human dopamine transporter after 120 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from recombinant human norepinephrine transporter after 120 min by scintillation counting method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031340
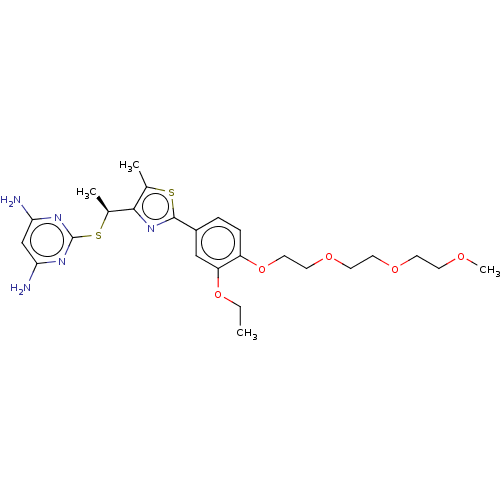
(CHEMBL3358095)Show SMILES CCOc1cc(ccc1OCCOCCOCCOC)-c1nc([C@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C25H35N5O5S2/c1-5-34-20-14-18(6-7-19(20)35-13-12-33-11-10-32-9-8-31-4)24-30-23(16(2)36-24)17(3)37-25-28-21(26)15-22(27)29-25/h6-7,14-15,17H,5,8-13H2,1-4H3,(H4,26,27,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse Nav1.7 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451457
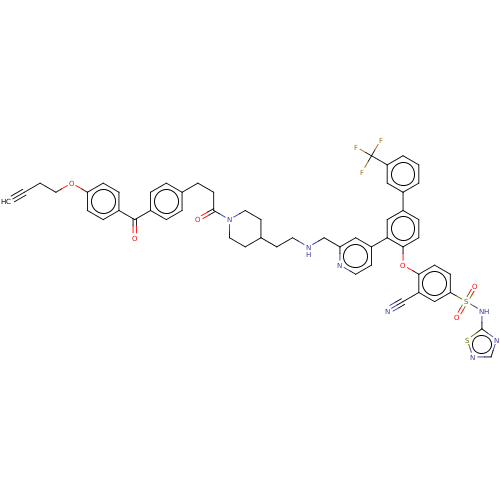
(CHEMBL4210135)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCN(CC2)C(=O)CCc2ccc(cc2)C(=O)c2ccc(OCCC#C)cc2)c1 Show InChI InChI=1S/C55H48F3N7O6S2/c1-2-3-29-70-47-15-12-40(13-16-47)53(67)39-10-7-37(8-11-39)9-20-52(66)65-27-23-38(24-28-65)21-25-60-35-46-31-43(22-26-61-46)49-33-42(41-5-4-6-45(30-41)55(56,57)58)14-18-51(49)71-50-19-17-48(32-44(50)34-59)73(68,69)64-54-62-36-63-72-54/h1,4-8,10-19,22,26,30-33,36,38,60H,3,9,20-21,23-25,27-29,35H2,(H,62,63,64) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451458
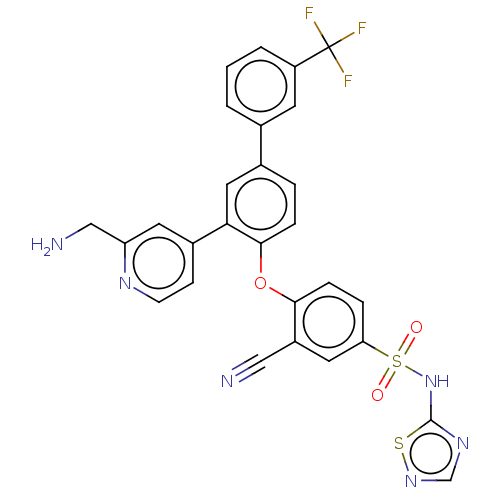
(CHEMBL4205773)Show SMILES NCc1cc(ccn1)-c1cc(ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C28H19F3N6O3S2/c29-28(30,31)21-3-1-2-17(10-21)18-4-6-26(24(13-18)19-8-9-34-22(11-19)15-33)40-25-7-5-23(12-20(25)14-32)42(38,39)37-27-35-16-36-41-27/h1-13,16H,15,33H2,(H,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451456
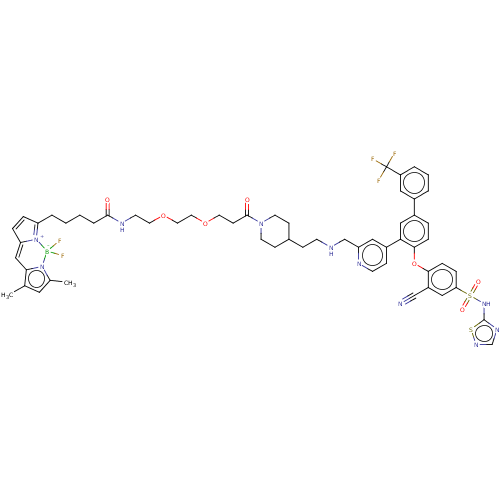
(CHEMBL4215058)Show SMILES Cc1cc(C)n2c1C=C1C=CC(CCCCC(=O)NCCOCCOCCC(=O)N3CCC(CCNCc4cc(ccn4)-c4cc(ccc4Oc4ccc(cc4C#N)S(=O)(=O)Nc4ncns4)-c4cccc(c4)C(F)(F)F)CC3)=[N+]1[B-]2(F)F |c:10,85,t:8| Show InChI InChI=1S/C58H62BF5N10O7S2/c1-39-30-40(2)73-52(39)35-49-12-11-48(74(49)59(73,63)64)8-3-4-9-55(75)68-23-27-80-29-28-79-26-20-56(76)72-24-18-41(19-25-72)16-21-66-37-47-32-44(17-22-67-47)51-34-43(42-6-5-7-46(31-42)58(60,61)62)10-14-54(51)81-53-15-13-50(33-45(53)36-65)83(77,78)71-57-69-38-70-82-57/h5-7,10-15,17,22,30-35,38,41,66H,3-4,8-9,16,18-21,23-29,37H2,1-2H3,(H,68,75)(H,69,70,71) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451458

(CHEMBL4205773)Show SMILES NCc1cc(ccn1)-c1cc(ccc1Oc1ccc(cc1C#N)S(=O)(=O)Nc1ncns1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C28H19F3N6O3S2/c29-28(30,31)21-3-1-2-17(10-21)18-4-6-26(24(13-18)19-8-9-34-22(11-19)15-33)40-25-7-5-23(12-20(25)14-32)42(38,39)37-27-35-16-36-41-27/h1-13,16H,15,33H2,(H,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451454
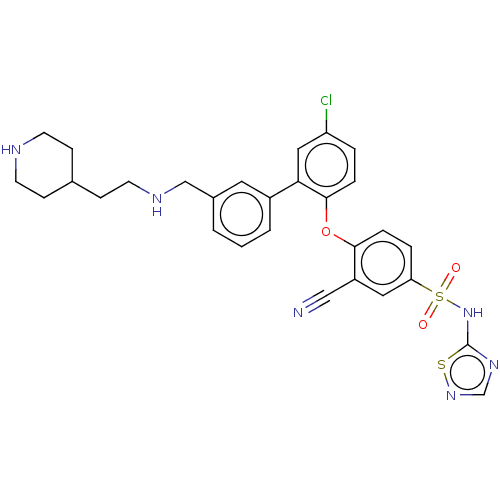
(CHEMBL4207534)Show SMILES Clc1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1cccc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C29H29ClN6O3S2/c30-24-4-6-28(39-27-7-5-25(15-23(27)17-31)41(37,38)36-29-34-19-35-40-29)26(16-24)22-3-1-2-21(14-22)18-33-13-10-20-8-11-32-12-9-20/h1-7,14-16,19-20,32-33H,8-13,18H2,(H,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440179

(CHEMBL2426567)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OCCO)c(OCCO)c1 Show InChI InChI=1S/C21H27N5O4S2/c1-2-3-17-14(12-31-21-25-18(22)11-19(23)26-21)24-20(32-17)13-4-5-15(29-8-6-27)16(10-13)30-9-7-28/h4-5,10-11,27-28H,2-3,6-9,12H2,1H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of [3H]-dC uptake by scintillation counting analysis |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440183
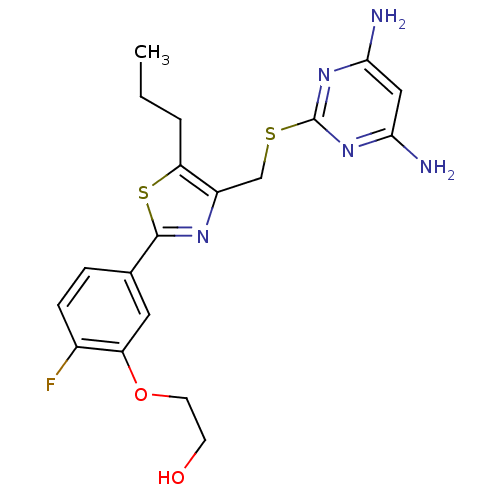
(CHEMBL2426563)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(F)c(OCCO)c1 Show InChI InChI=1S/C19H22FN5O2S2/c1-2-3-15-13(10-28-19-24-16(21)9-17(22)25-19)23-18(29-15)11-4-5-12(20)14(8-11)27-7-6-26/h4-5,8-9,26H,2-3,6-7,10H2,1H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440176

(CHEMBL2426570)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCO)c1 Show InChI InChI=1S/C20H25N5O3S2/c1-3-4-16-13(11-29-20-24-17(21)10-18(22)25-20)23-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-26/h5-6,9-10,26H,3-4,7-8,11H2,1-2H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of [3H]-dC uptake by scintillation counting analysis |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440176

(CHEMBL2426570)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCO)c1 Show InChI InChI=1S/C20H25N5O3S2/c1-3-4-16-13(11-29-20-24-17(21)10-18(22)25-20)23-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-26/h5-6,9-10,26H,3-4,7-8,11H2,1-2H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031339

(CHEMBL3358096)Show SMILES CCOc1cc(ccc1OCCOCCOCCOC)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C25H35N5O5S2/c1-5-34-20-14-18(6-7-19(20)35-13-12-33-11-10-32-9-8-31-4)24-30-23(16(2)36-24)17(3)37-25-28-21(26)15-22(27)29-25/h6-7,14-15,17H,5,8-13H2,1-4H3,(H4,26,27,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of [3H]-dC uptake by scintillation counting analysis |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173

(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of [3H]-dC uptake by scintillation counting analysis |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173

(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451459
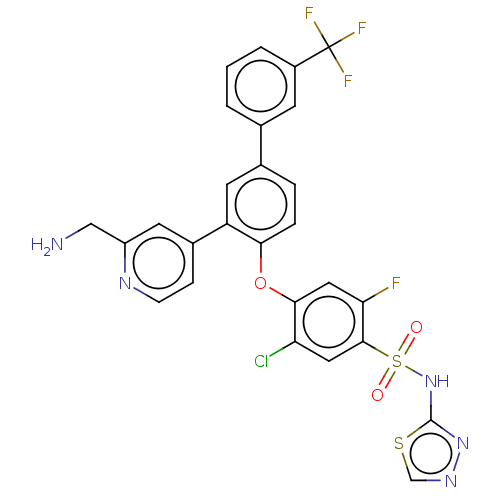
(CHEMBL4208664)Show SMILES NCc1cc(ccn1)-c1cc(ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1nncs1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H18ClF4N5O3S2/c28-21-11-25(42(38,39)37-26-36-35-14-41-26)22(29)12-24(21)40-23-5-4-16(15-2-1-3-18(8-15)27(30,31)32)10-20(23)17-6-7-34-19(9-17)13-33/h1-12,14H,13,33H2,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440177
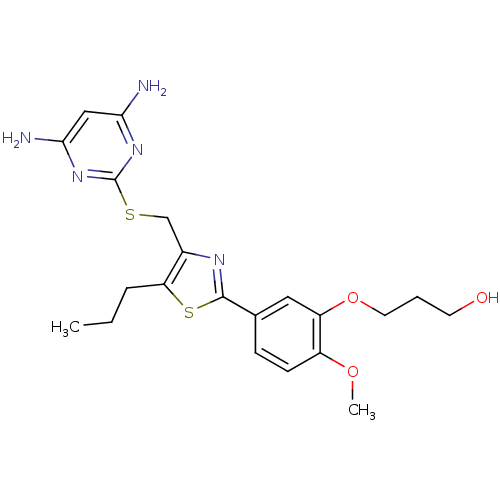
(CHEMBL2426569)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCCO)c1 Show InChI InChI=1S/C21H27N5O3S2/c1-3-5-17-14(12-30-21-25-18(22)11-19(23)26-21)24-20(31-17)13-6-7-15(28-2)16(10-13)29-9-4-8-27/h6-7,10-11,27H,3-5,8-9,12H2,1-2H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50451453
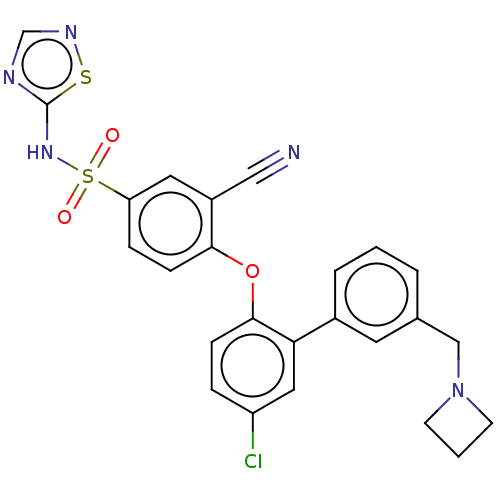
(CHEMBL2325603)Show SMILES Clc1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1cccc(CN2CCC2)c1 Show InChI InChI=1S/C25H20ClN5O3S2/c26-20-5-7-24(22(13-20)18-4-1-3-17(11-18)15-31-9-2-10-31)34-23-8-6-21(12-19(23)14-27)36(32,33)30-25-28-16-29-35-25/h1,3-8,11-13,16H,2,9-10,15H2,(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440184
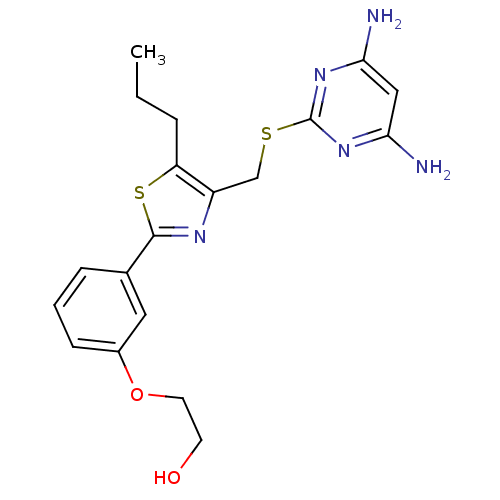
(CHEMBL2426562)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1cccc(OCCO)c1 Show InChI InChI=1S/C19H23N5O2S2/c1-2-4-15-14(11-27-19-23-16(20)10-17(21)24-19)22-18(28-15)12-5-3-6-13(9-12)26-8-7-25/h3,5-6,9-10,25H,2,4,7-8,11H2,1H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Homo sapiens (Human)) | BDBM50451447

(CHEMBL4217988)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2ncns2)c(c1)-c1ccnc(CNCCC2CCNCC2)c1 Show InChI InChI=1S/C35H32F3N7O3S2/c36-35(37,38)28-3-1-2-24(16-28)25-4-6-33(48-32-7-5-30(18-27(32)20-39)50(46,47)45-34-43-22-44-49-34)31(19-25)26-11-15-42-29(17-26)21-41-14-10-23-8-12-40-13-9-23/h1-7,11,15-19,22-23,40-41H,8-10,12-14,21H2,(H,43,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.2 expressed in HEK cells by patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 4805-4811 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.056
BindingDB Entry DOI: 10.7270/Q25Q4ZN1 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440179

(CHEMBL2426567)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OCCO)c(OCCO)c1 Show InChI InChI=1S/C21H27N5O4S2/c1-2-3-17-14(12-31-21-25-18(22)11-19(23)26-21)24-20(32-17)13-4-5-15(29-8-6-27)16(10-13)30-9-7-28/h4-5,10-11,27-28H,2-3,6-9,12H2,1H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440182
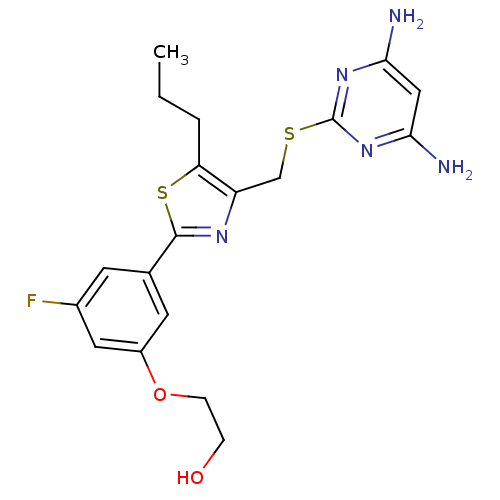
(CHEMBL2426564)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1cc(F)cc(OCCO)c1 Show InChI InChI=1S/C19H22FN5O2S2/c1-2-3-15-14(10-28-19-24-16(21)9-17(22)25-19)23-18(29-15)11-6-12(20)8-13(7-11)27-5-4-26/h6-9,26H,2-5,10H2,1H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440175
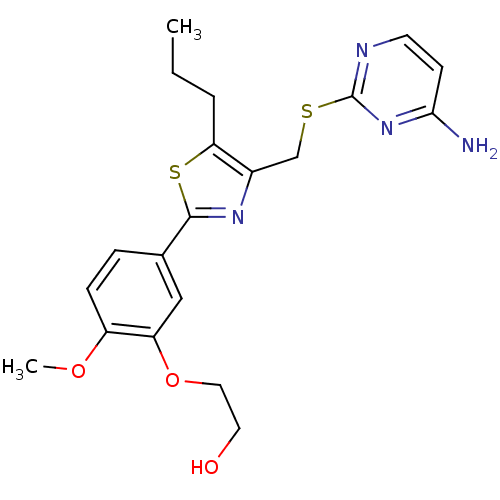
(CHEMBL2426571)Show SMILES CCCc1sc(nc1CSc1nccc(N)n1)-c1ccc(OC)c(OCCO)c1 Show InChI InChI=1S/C20H24N4O3S2/c1-3-4-17-14(12-28-20-22-8-7-18(21)24-20)23-19(29-17)13-5-6-15(26-2)16(11-13)27-10-9-25/h5-8,11,25H,3-4,9-10,12H2,1-2H3,(H2,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440174
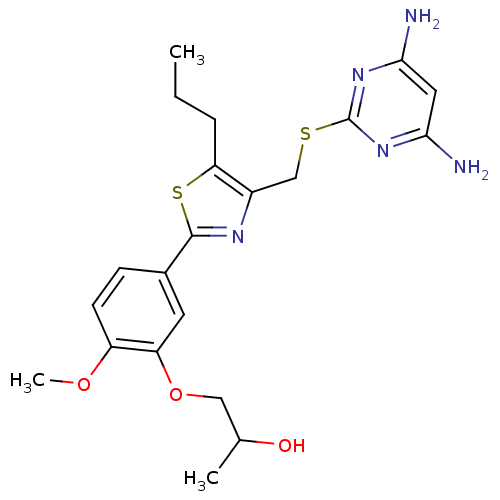
(CHEMBL2426572)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)O)c1 Show InChI InChI=1S/C21H27N5O3S2/c1-4-5-17-14(11-30-21-25-18(22)9-19(23)26-21)24-20(31-17)13-6-7-15(28-3)16(8-13)29-10-12(2)27/h6-9,12,27H,4-5,10-11H2,1-3H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Mus musculus) | BDBM50440151

(CHEMBL2426558)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C20H24FN5O2S2/c1-3-4-16-13(11-29-20-25-17(22)10-18(23)26-20)24-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-21/h5-6,9-10H,3-4,7-8,11H2,1-2H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in mouse L1210 cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440151

(CHEMBL2426558)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C20H24FN5O2S2/c1-3-4-16-13(11-29-20-25-17(22)10-18(23)26-20)24-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-21/h5-6,9-10H,3-4,7-8,11H2,1-2H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of tritiated deoxycytidine [3H]-dC uptake |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031337

(CHEMBL3358097)Show SMILES COc1ccc(cc1OCCNS(C)(=O)=O)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C20H26N6O4S3/c1-11-18(12(2)32-20-24-16(21)10-17(22)25-20)26-19(31-11)13-5-6-14(29-3)15(9-13)30-8-7-23-33(4,27)28/h5-6,9-10,12,23H,7-8H2,1-4H3,(H4,21,22,24,25)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of dCK in human CCRF-CEM cells assessed as inhibition of [3H]-dC uptake by scintillation counting analysis |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A in bovine brain mitochondria using serotonin as substrate preincubated for 30 mins measured after 30 mins by spectrofluorimetric ... |
Bioorg Med Chem Lett 25: 5281-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.048
BindingDB Entry DOI: 10.7270/Q2M90BH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data