 Found 60 hits with Last Name = 'alexandrova' and Initial = 'l'
Found 60 hits with Last Name = 'alexandrova' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477491

(CHEMBL398034)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H19Cl2N3O3S/c19-13-3-1-4-14(20)18(13)27(24,25)23-11-12-26-17-15(5-2-6-16(17)23)22-9-7-21-8-10-22/h1-6,21H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477488
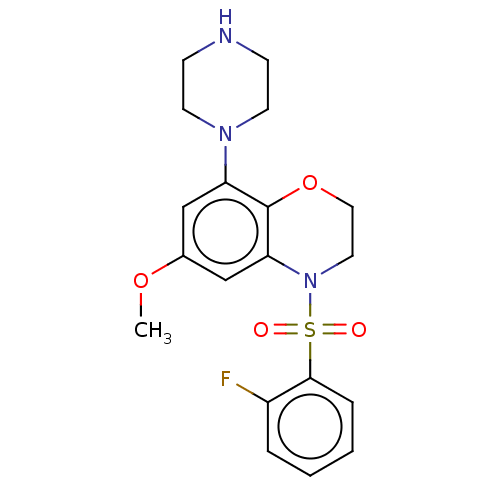
(CHEMBL394690)Show SMILES COc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H22FN3O4S/c1-26-14-12-16(22-8-6-21-7-9-22)19-17(13-14)23(10-11-27-19)28(24,25)18-5-3-2-4-15(18)20/h2-5,12-13,21H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477473

(CHEMBL246918)Show SMILES CC1(C)CN(c2cccc(N3CCNCC3)c2O1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H24FN3O3S/c1-20(2)14-24(28(25,26)18-9-4-3-6-15(18)21)17-8-5-7-16(19(17)27-20)23-12-10-22-11-13-23/h3-9,22H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477475

(CHEMBL246499)Show SMILES Cc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H22FN3O3S/c1-14-12-16(22-8-6-21-7-9-22)19-17(13-14)23(10-11-26-19)27(24,25)18-5-3-2-4-15(18)20/h2-5,12-13,21H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477479
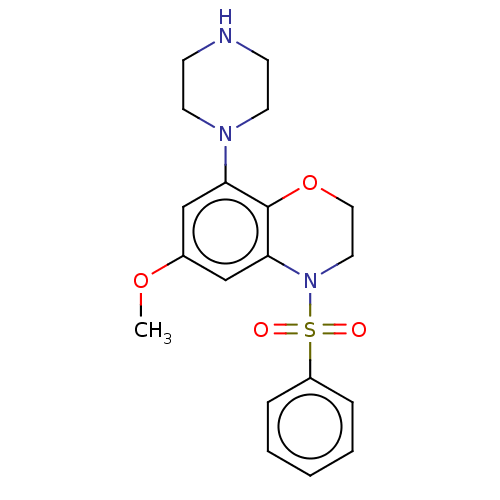
(CHEMBL246706)Show SMILES COc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H23N3O4S/c1-25-15-13-17(21-9-7-20-8-10-21)19-18(14-15)22(11-12-26-19)27(23,24)16-5-3-2-4-6-16/h2-6,13-14,20H,7-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477489

(CHEMBL246705)Show SMILES Fc1ccccc1S(=O)(=O)N1CCOc2c(cc(Cl)cc12)N1CCNCC1 Show InChI InChI=1S/C18H19ClFN3O3S/c19-13-11-15(22-7-5-21-6-8-22)18-16(12-13)23(9-10-26-18)27(24,25)17-4-2-1-3-14(17)20/h1-4,11-12,21H,5-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477477
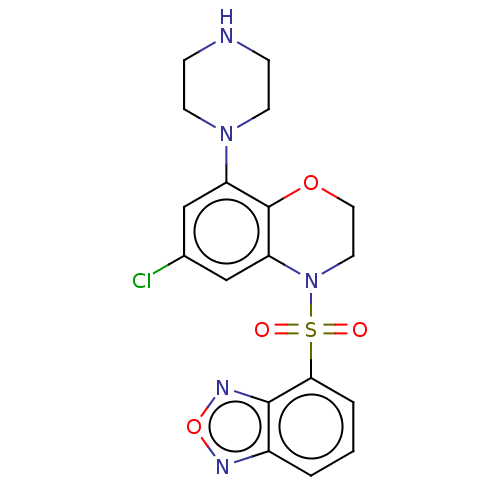
(CHEMBL246291)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2nonc12 Show InChI InChI=1S/C18H18ClN5O4S/c19-12-10-14(23-6-4-20-5-7-23)18-15(11-12)24(8-9-27-18)29(25,26)16-3-1-2-13-17(16)22-28-21-13/h1-3,10-11,20H,4-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477487

(CHEMBL246704)Show SMILES Fc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C18H19F2N3O3S/c19-13-11-15(22-7-5-21-6-8-22)18-16(12-13)23(9-10-26-18)27(24,25)17-4-2-1-3-14(17)20/h1-4,11-12,21H,5-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477497
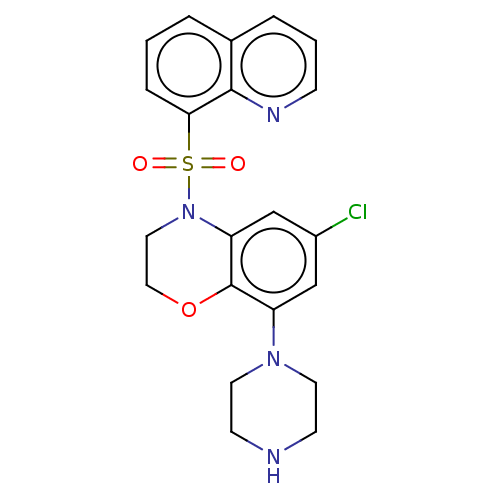
(CHEMBL246498)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2cccnc12 Show InChI InChI=1S/C21H21ClN4O3S/c22-16-13-17(25-9-7-23-8-10-25)21-18(14-16)26(11-12-29-21)30(27,28)19-5-1-3-15-4-2-6-24-20(15)19/h1-6,13-14,23H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477490
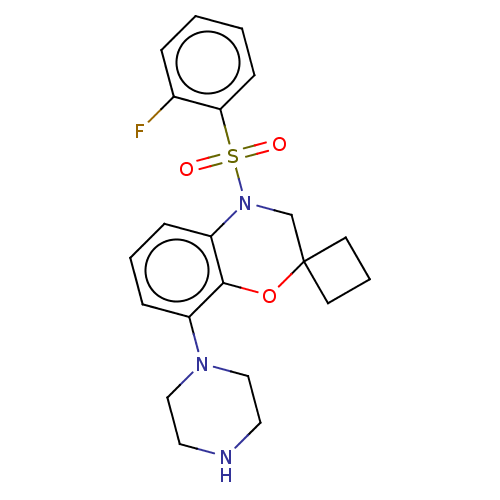
(CHEMBL394691)Show SMILES Fc1ccccc1S(=O)(=O)N1CC2(CCC2)Oc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H24FN3O3S/c22-16-5-1-2-8-19(16)29(26,27)25-15-21(9-4-10-21)28-20-17(6-3-7-18(20)25)24-13-11-23-12-14-24/h1-3,5-8,23H,4,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477472
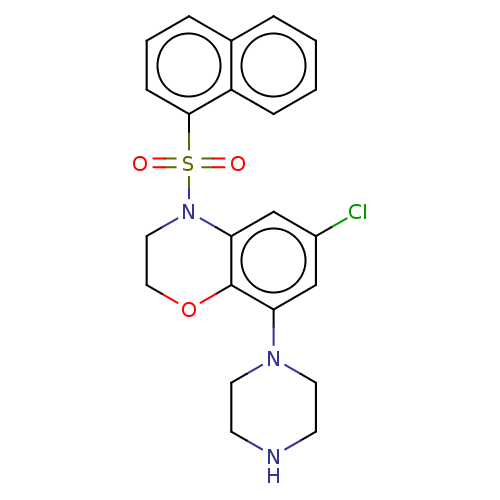
(CHEMBL398036)Show SMILES Clc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22ClN3O3S/c23-17-14-19(25-10-8-24-9-11-25)22-20(15-17)26(12-13-29-22)30(27,28)21-7-3-5-16-4-1-2-6-18(16)21/h1-7,14-15,24H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477483
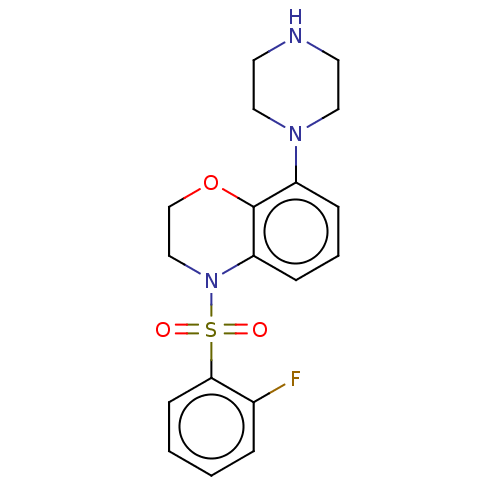
(CHEMBL246078)Show InChI InChI=1S/C18H20FN3O3S/c19-14-4-1-2-7-17(14)26(23,24)22-12-13-25-18-15(5-3-6-16(18)22)21-10-8-20-9-11-21/h1-7,20H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477471

(CHEMBL246917)Show SMILES Nc1ccc(cc1)S(=O)(=O)N1CCOc2c(cc(F)cc12)N1CCNCC1 Show InChI InChI=1S/C18H21FN4O3S/c19-13-11-16(22-7-5-21-6-8-22)18-17(12-13)23(9-10-26-18)27(24,25)15-3-1-14(20)2-4-15/h1-4,11-12,21H,5-10,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477478

(CHEMBL246500)Show SMILES Cc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1C#N Show InChI InChI=1S/C20H22N4O3S/c1-15-12-17(23-8-6-22-7-9-23)20-18(13-15)24(10-11-27-20)28(25,26)19-5-3-2-4-16(19)14-21/h2-5,12-13,22H,6-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477494
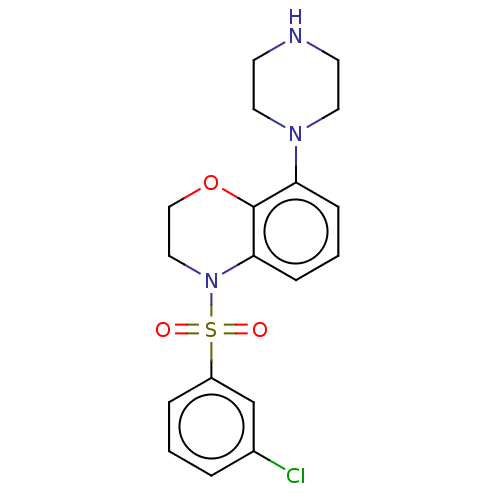
(CHEMBL246079)Show SMILES Clc1cccc(c1)S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H20ClN3O3S/c19-14-3-1-4-15(13-14)26(23,24)22-11-12-25-18-16(5-2-6-17(18)22)21-9-7-20-8-10-21/h1-6,13,20H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477470
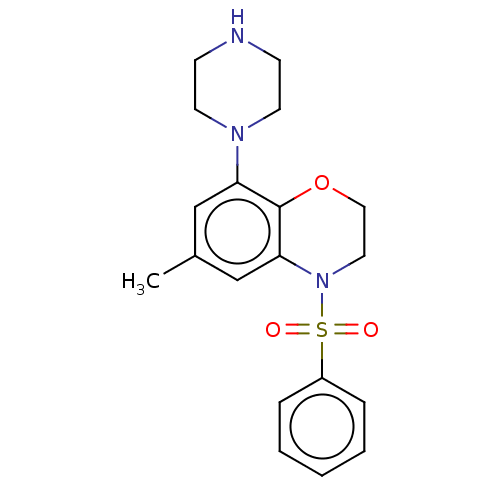
(CHEMBL394689)Show SMILES Cc1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H23N3O3S/c1-15-13-17(21-9-7-20-8-10-21)19-18(14-15)22(11-12-25-19)26(23,24)16-5-3-2-4-6-16/h2-6,13-14,20H,7-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477492

(CHEMBL398035)Show SMILES Clc1ccccc1S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H20ClN3O3S/c19-14-4-1-2-7-17(14)26(23,24)22-12-13-25-18-15(5-3-6-16(18)22)21-10-8-20-9-11-21/h1-7,20H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477474

(CHEMBL247121)Show SMILES CC1(C)CN(c2cc(F)cc(C3CCNCC3)c2O1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H25FN2O3S/c1-21(2)14-24(28(25,26)17-6-4-3-5-7-17)19-13-16(22)12-18(20(19)27-21)15-8-10-23-11-9-15/h3-7,12-13,15,23H,8-11,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477495
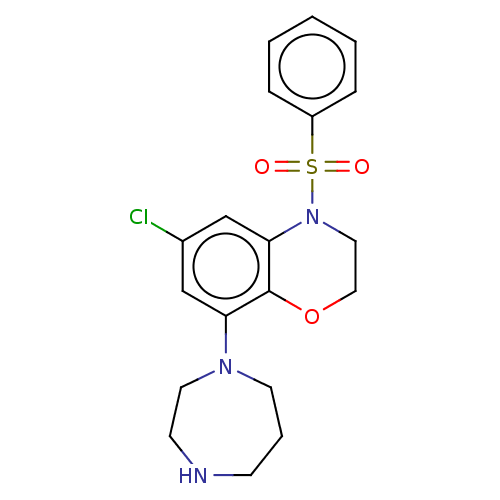
(CHEMBL394949)Show SMILES Clc1cc2N(CCOc2c(c1)N1CCCNCC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H22ClN3O3S/c20-15-13-17(22-9-4-7-21-8-10-22)19-18(14-15)23(11-12-26-19)27(24,25)16-5-2-1-3-6-16/h1-3,5-6,13-14,21H,4,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477496

(CHEMBL438675)Show SMILES CC1(C)CN(c2cc(F)cc(C3CCNCC3)c2O1)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C21H24F2N2O3S/c1-21(2)13-25(29(26,27)17-5-3-4-15(22)10-17)19-12-16(23)11-18(20(19)28-21)14-6-8-24-9-7-14/h3-5,10-12,14,24H,6-9,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477486

(CHEMBL246916)Show SMILES CC(C)(C)c1cc(N2CCNCC2)c2OCCN(c2c1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H28FN3O3S/c1-22(2,3)16-14-18(25-10-8-24-9-11-25)21-19(15-16)26(12-13-29-21)30(27,28)20-7-5-4-6-17(20)23/h4-7,14-15,24H,8-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477480
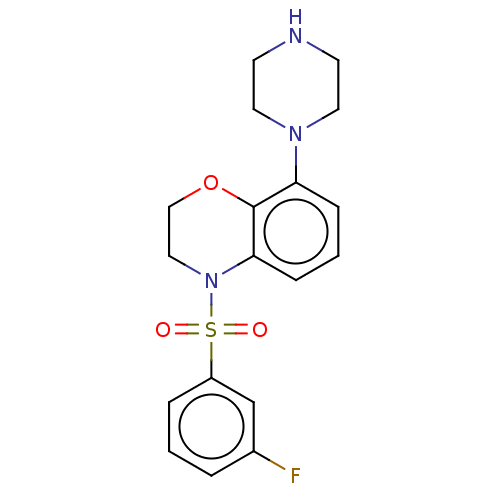
(CHEMBL246080)Show SMILES Fc1cccc(c1)S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H20FN3O3S/c19-14-3-1-4-15(13-14)26(23,24)22-11-12-25-18-16(5-2-6-17(18)22)21-9-7-20-8-10-21/h1-6,13,20H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477493
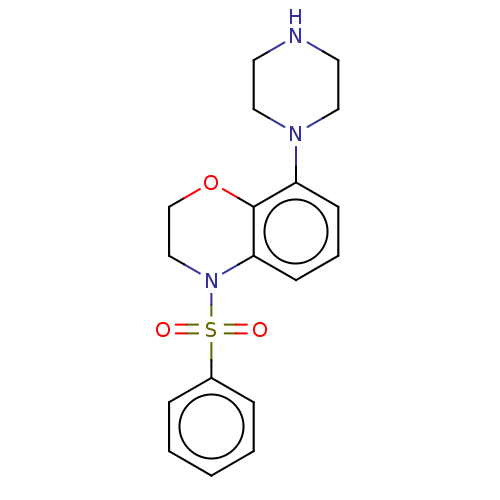
(CHEMBL246077)Show InChI InChI=1S/C18H21N3O3S/c22-25(23,15-5-2-1-3-6-15)21-13-14-24-18-16(7-4-8-17(18)21)20-11-9-19-10-12-20/h1-8,19H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477482

(CHEMBL247120)Show SMILES CC1CN(CC(C)N1)c1cc(C)cc2N(CCOc12)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H26ClN3O3S/c1-14-10-19(24-12-15(2)23-16(3)13-24)21-20(11-14)25(8-9-28-21)29(26,27)18-6-4-17(22)5-7-18/h4-7,10-11,15-16,23H,8-9,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477481
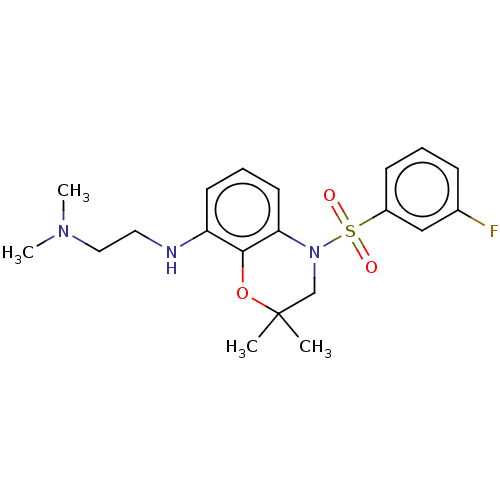
(CHEMBL247325)Show SMILES CN(C)CCNc1cccc2N(CC(C)(C)Oc12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C20H26FN3O3S/c1-20(2)14-24(28(25,26)16-8-5-7-15(21)13-16)18-10-6-9-17(19(18)27-20)22-11-12-23(3)4/h5-10,13,22H,11-12,14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477485
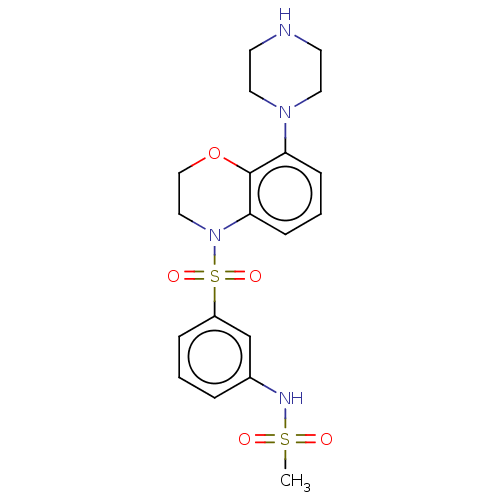
(CHEMBL246289)Show SMILES CS(=O)(=O)Nc1cccc(c1)S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C19H24N4O5S2/c1-29(24,25)21-15-4-2-5-16(14-15)30(26,27)23-12-13-28-19-17(6-3-7-18(19)23)22-10-8-20-9-11-22/h2-7,14,20-21H,8-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477476
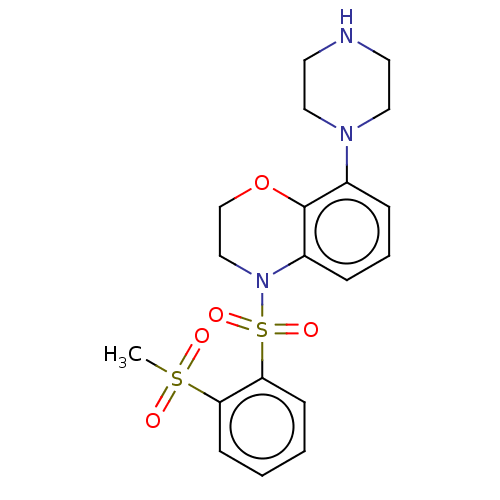
(CHEMBL246290)Show SMILES CS(=O)(=O)c1ccccc1S(=O)(=O)N1CCOc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C19H23N3O5S2/c1-28(23,24)17-7-2-3-8-18(17)29(25,26)22-13-14-27-19-15(5-4-6-16(19)22)21-11-9-20-10-12-21/h2-8,20H,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50477484
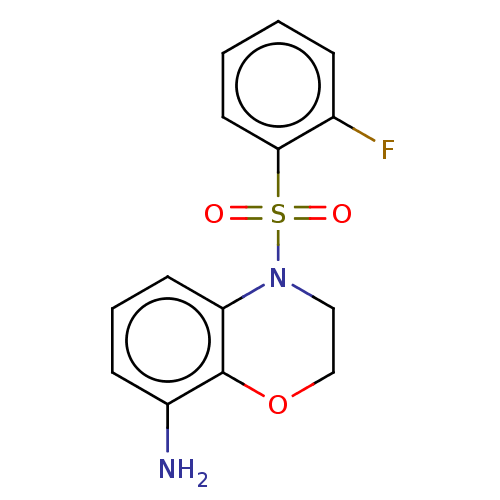
(CHEMBL247119)Show InChI InChI=1S/C14H13FN2O3S/c15-10-4-1-2-7-13(10)21(18,19)17-8-9-20-14-11(16)5-3-6-12(14)17/h1-7H,8-9,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 3504-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.093
BindingDB Entry DOI: 10.7270/Q29P34FX |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300504

(CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...)Show SMILES CN(Cc1cccc2C(NS(=O)(=O)c12)=C1C(=O)[C@@H](N(Cc2ccc(F)cc2)C1=O)C(C)(C)C)S(N)(=O)=O |r,w:14.16| Show InChI InChI=1S/C24H27FN4O6S2/c1-24(2,3)22-20(30)18(23(31)29(22)12-14-8-10-16(25)11-9-14)19-17-7-5-6-15(13-28(4)37(26,34)35)21(17)36(32,33)27-19/h5-11,22,27H,12-13H2,1-4H3,(H2,26,34,35)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301911

(CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1Cl |r,t:21| Show InChI InChI=1S/C24H25ClFN3O6S2/c1-24(2,3)21-20(30)18(23(31)29(21)12-13-5-7-14(26)8-6-13)19-22(25)37(34,35)17-11-15(28-36(4,32)33)9-10-16(17)27-19/h5-11,18,21-22,28H,12H2,1-4H3/t18?,21-,22?/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300502

(CHEMBL572682 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C2NS(=O)(=O)c3c2cccc3C=NS(N)(=O)=O)C1=O |r,w:28.30| Show InChI InChI=1S/C23H25FN4O6S2/c1-23(2,3)21-19(29)17(22(30)28(21)12-13-7-9-15(24)10-8-13)18-16-6-4-5-14(11-26-36(25,33)34)20(16)35(31,32)27-18/h4-11,17-18,21,27H,12H2,1-3H3,(H2,25,33,34)/t17?,18?,21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300497
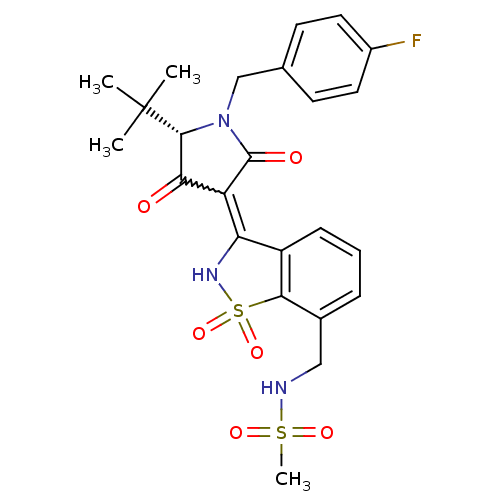
(CHEMBL578433 | N-({3-[(5S)-5-tert-butyl-1-(4-fluor...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)=C1NS(=O)(=O)c2c1cccc2CNS(C)(=O)=O |r,w:16.17| Show InChI InChI=1S/C24H26FN3O6S2/c1-24(2,3)22-20(29)18(23(30)28(22)13-14-8-10-16(25)11-9-14)19-17-7-5-6-15(12-26-35(4,31)32)21(17)36(33,34)27-19/h5-11,22,26-27H,12-13H2,1-4H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
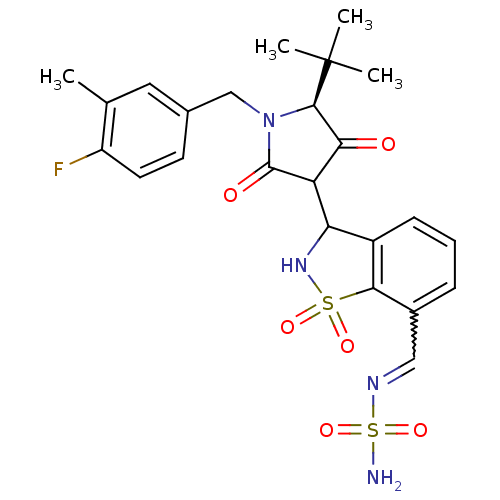
(Hepatitis C virus (HCV)) | BDBM50300503
(CHEMBL582995 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...)Show SMILES Cc1cc(CN2[C@H](C(=O)C(C3NS(=O)(=O)c4c3cccc4C=NS(N)(=O)=O)C2=O)C(C)(C)C)ccc1F |r,w:21.22| Show InChI InChI=1S/C24H27FN4O6S2/c1-13-10-14(8-9-17(13)25)12-29-22(24(2,3)4)20(30)18(23(29)31)19-16-7-5-6-15(11-27-37(26,34)35)21(16)36(32,33)28-19/h5-11,18-19,22,28H,12H2,1-4H3,(H2,26,34,35)/t18?,19?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301902

(CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES Cc1cc(CN2[C@H](C(=O)C(C2=O)C2=Nc3ccc(NS(C)(=O)=O)cc3S(=O)(=O)C2)C(C)(C)C)ccc1F |r,t:13| Show InChI InChI=1S/C25H28FN3O6S2/c1-14-10-15(6-8-17(14)26)12-29-23(25(2,3)4)22(30)21(24(29)31)19-13-37(34,35)20-11-16(28-36(5,32)33)7-9-18(20)27-19/h6-11,21,23,28H,12-13H2,1-5H3/t21?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300499
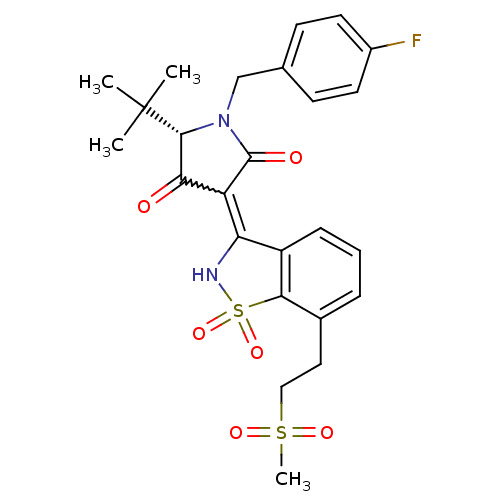
((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-[...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)=C1NS(=O)(=O)c2c1cccc2CCS(C)(=O)=O |r,w:16.17| Show InChI InChI=1S/C25H27FN2O6S2/c1-25(2,3)23-21(29)19(24(30)28(23)14-15-8-10-17(26)11-9-15)20-18-7-5-6-16(12-13-35(4,31)32)22(18)36(33,34)27-20/h5-11,23,27H,12-14H2,1-4H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
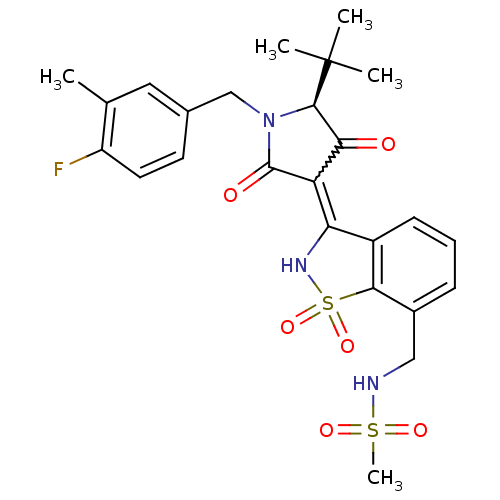
(Hepatitis C virus (HCV)) | BDBM50300498
(CHEMBL575777 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES Cc1cc(CN2[C@H](C(=O)C(C2=O)=C2NS(=O)(=O)c3c2cccc3CNS(C)(=O)=O)C(C)(C)C)ccc1F |r,w:9.8| Show InChI InChI=1S/C25H28FN3O6S2/c1-14-11-15(9-10-18(14)26)13-29-23(25(2,3)4)21(30)19(24(29)31)20-17-8-6-7-16(12-27-36(5,32)33)22(17)37(34,35)28-20/h6-11,23,27-28H,12-13H2,1-5H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301903

(CHEMBL569120 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES COc1cc(CN2[C@H](C(=O)C(C2=O)C2=Nc3ccc(NS(C)(=O)=O)cc3S(=O)(=O)C2)C(C)(C)C)ccc1F |r,t:14| Show InChI InChI=1S/C25H28FN3O7S2/c1-25(2,3)23-22(30)21(24(31)29(23)12-14-6-8-16(26)19(10-14)36-4)18-13-38(34,35)20-11-15(28-37(5,32)33)7-9-17(20)27-18/h6-11,21,23,28H,12-13H2,1-5H3/t21?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301914
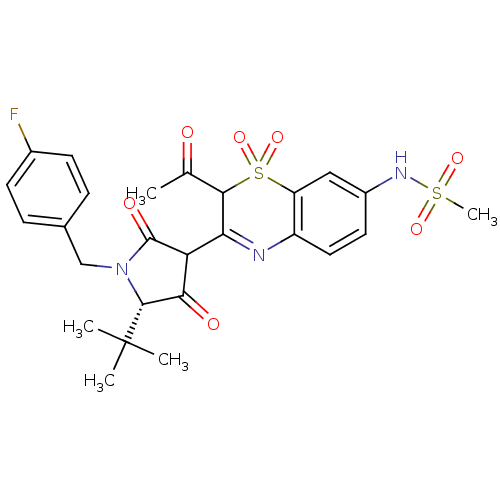
(CHEMBL570249 | N-{2-Acetyl-3-[(S)-5-tert-butyl-1-(...)Show SMILES CC(=O)C1C(=Nc2ccc(NS(C)(=O)=O)cc2S1(=O)=O)C1C(=O)[C@@H](N(Cc2ccc(F)cc2)C1=O)C(C)(C)C |r,c:4| Show InChI InChI=1S/C26H28FN3O7S2/c1-14(31)23-21(28-18-11-10-17(29-38(5,34)35)12-19(18)39(23,36)37)20-22(32)24(26(2,3)4)30(25(20)33)13-15-6-8-16(27)9-7-15/h6-12,20,23-24,29H,13H2,1-5H3/t20?,23?,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301906
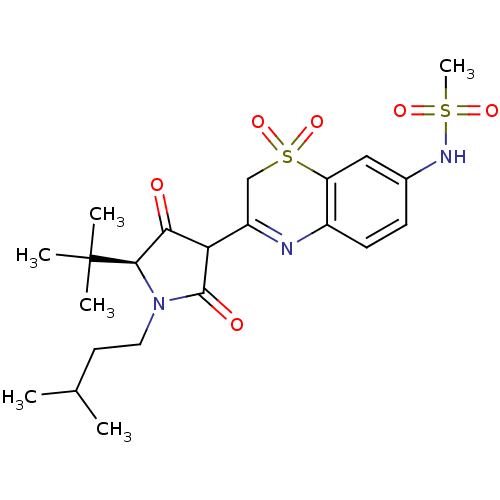
(CHEMBL571606 | N-{3-[(S)-5-tert-Butyl-4-hydroxy-1-...)Show SMILES CC(C)CCN1[C@H](C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1)C(C)(C)C |r,t:13| Show InChI InChI=1S/C22H31N3O6S2/c1-13(2)9-10-25-20(22(3,4)5)19(26)18(21(25)27)16-12-33(30,31)17-11-14(24-32(6,28)29)7-8-15(17)23-16/h7-8,11,13,18,20,24H,9-10,12H2,1-6H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301901
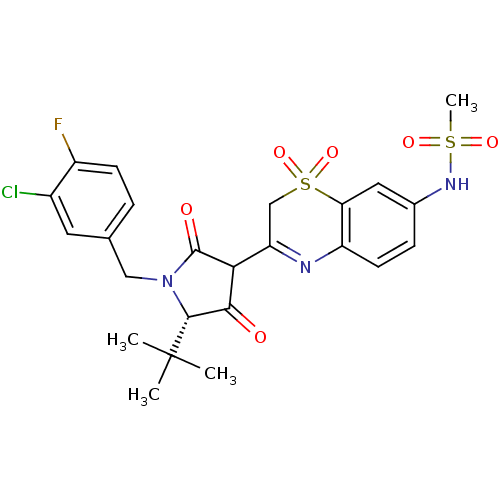
(CHEMBL570934 | N-{3-[(S)-5-tert-Butyl-1-(3-chloro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)c(Cl)c2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:22| Show InChI InChI=1S/C24H25ClFN3O6S2/c1-24(2,3)22-21(30)20(23(31)29(22)11-13-5-7-16(26)15(25)9-13)18-12-37(34,35)19-10-14(28-36(4,32)33)6-8-17(19)27-18/h5-10,20,22,28H,11-12H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300505
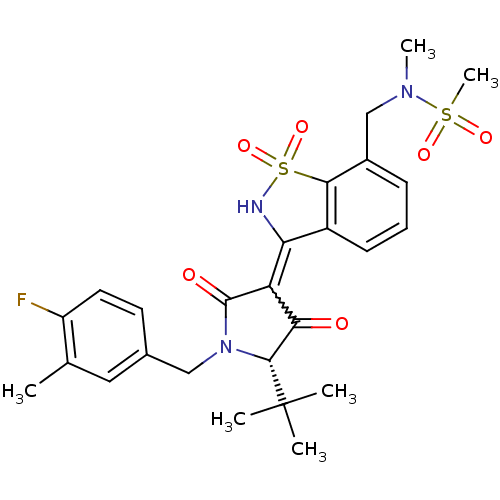
(CHEMBL572683 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CN(Cc1cccc2C(NS(=O)(=O)c12)=C1C(=O)[C@@H](N(Cc2ccc(F)c(C)c2)C1=O)C(C)(C)C)S(C)(=O)=O |r,w:14.16| Show InChI InChI=1S/C26H30FN3O6S2/c1-15-12-16(10-11-19(15)27)13-30-24(26(2,3)4)22(31)20(25(30)32)21-18-9-7-8-17(14-29(5)37(6,33)34)23(18)38(35,36)28-21/h7-12,24,28H,13-14H2,1-6H3/t24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300496

(CHEMBL577404 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)=C1NS(=O)(=O)c2c1cccc2NS(C)(=O)=O |r,w:16.17| Show InChI InChI=1S/C23H24FN3O6S2/c1-23(2,3)21-19(28)17(22(29)27(21)12-13-8-10-14(24)11-9-13)18-15-6-5-7-16(25-34(4,30)31)20(15)35(32,33)26-18/h5-11,21,25-26H,12H2,1-4H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301913

(CHEMBL571564 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1C#N |r,t:21| Show InChI InChI=1S/C25H25FN4O6S2/c1-25(2,3)23-22(31)20(24(32)30(23)13-14-5-7-15(26)8-6-14)21-19(12-27)38(35,36)18-11-16(29-37(4,33)34)9-10-17(18)28-21/h5-11,19-20,23,29H,13H2,1-4H3/t19?,20?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300501
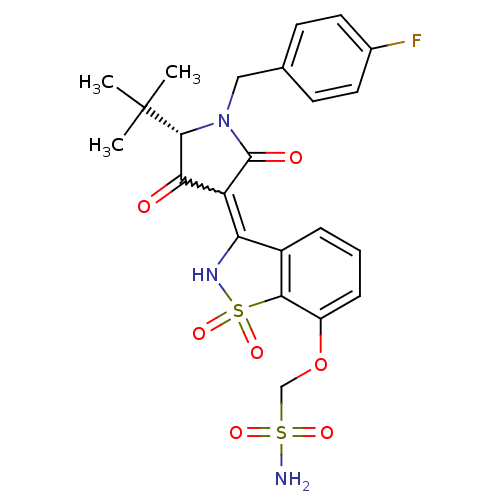
(CHEMBL573175 | {3-[(S)-5-tert-Butyl-1-(4-fluoro-be...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)=C1NS(=O)(=O)c2c1cccc2OCS(N)(=O)=O |r,w:16.17| Show InChI InChI=1S/C23H24FN3O7S2/c1-23(2,3)21-19(28)17(22(29)27(21)11-13-7-9-14(24)10-8-13)18-15-5-4-6-16(34-12-35(25,30)31)20(15)36(32,33)26-18/h4-10,21,26H,11-12H2,1-3H3,(H2,25,30,31)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301900
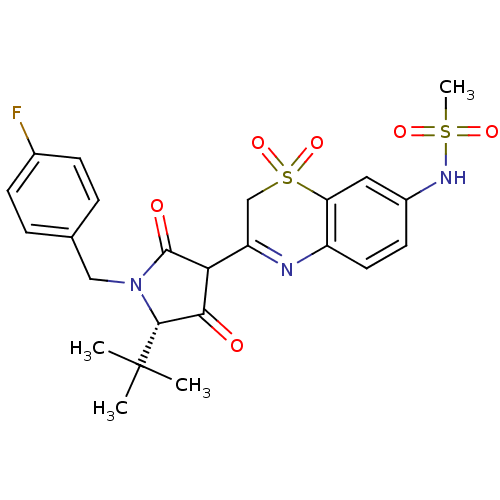
(CHEMBL568894 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:21| Show InChI InChI=1S/C24H26FN3O6S2/c1-24(2,3)22-21(29)20(23(30)28(22)12-14-5-7-15(25)8-6-14)18-13-36(33,34)19-11-16(27-35(4,31)32)9-10-17(19)26-18/h5-11,20,22,27H,12-13H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301907
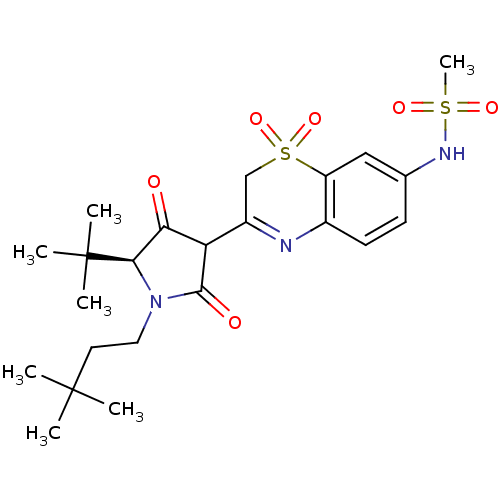
(CHEMBL572246 | N-{3-[(S)-5-tert-Butyl-1-(3,3-dimet...)Show SMILES CC(C)(C)CCN1[C@H](C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1)C(C)(C)C |r,t:14| Show InChI InChI=1S/C23H33N3O6S2/c1-22(2,3)10-11-26-20(23(4,5)6)19(27)18(21(26)28)16-13-34(31,32)17-12-14(25-33(7,29)30)8-9-15(17)24-16/h8-9,12,18,20,25H,10-11,13H2,1-7H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301899
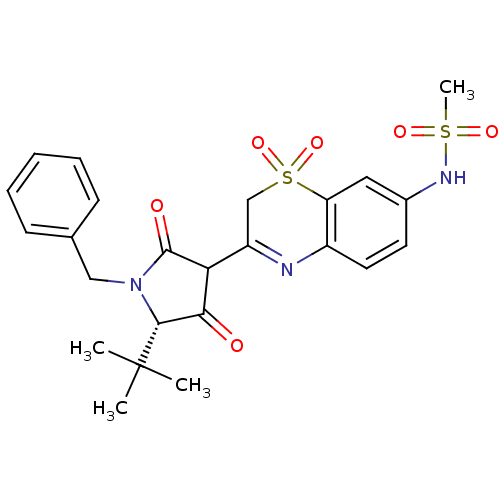
(CHEMBL585384 | N-[3-((S)-1-Benzyl-5-tert-butyl-4-h...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccccc2)C(=O)C(C1=O)C1=Nc2ccc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:20| Show InChI InChI=1S/C24H27N3O6S2/c1-24(2,3)22-21(28)20(23(29)27(22)13-15-8-6-5-7-9-15)18-14-35(32,33)19-12-16(26-34(4,30)31)10-11-17(19)25-18/h5-12,20,22,26H,13-14H2,1-4H3/t20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301916
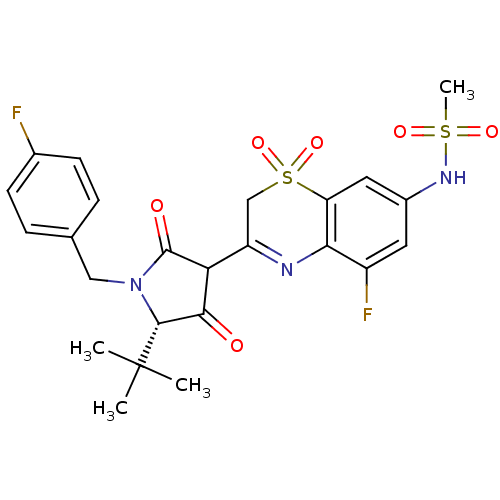
(CHEMBL585715 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)C1=Nc2c(F)cc(NS(C)(=O)=O)cc2S(=O)(=O)C1 |r,t:21| Show InChI InChI=1S/C24H25F2N3O6S2/c1-24(2,3)22-21(30)19(23(31)29(22)11-13-5-7-14(25)8-6-13)17-12-37(34,35)18-10-15(28-36(4,32)33)9-16(26)20(18)27-17/h5-10,19,22,28H,11-12H2,1-4H3/t19?,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300500
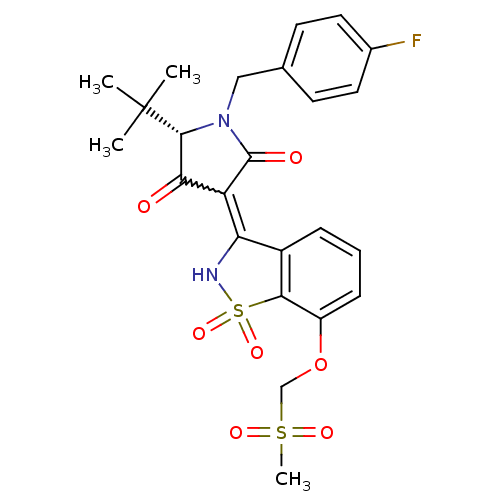
((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-(...)Show SMILES CC(C)(C)[C@@H]1N(Cc2ccc(F)cc2)C(=O)C(C1=O)=C1NS(=O)(=O)c2c1cccc2OCS(C)(=O)=O |r,w:16.17| Show InChI InChI=1S/C24H25FN2O7S2/c1-24(2,3)22-20(28)18(23(29)27(22)12-14-8-10-15(25)11-9-14)19-16-6-5-7-17(34-13-35(4,30)31)21(16)36(32,33)26-19/h5-11,22,26H,12-13H2,1-4H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50301912
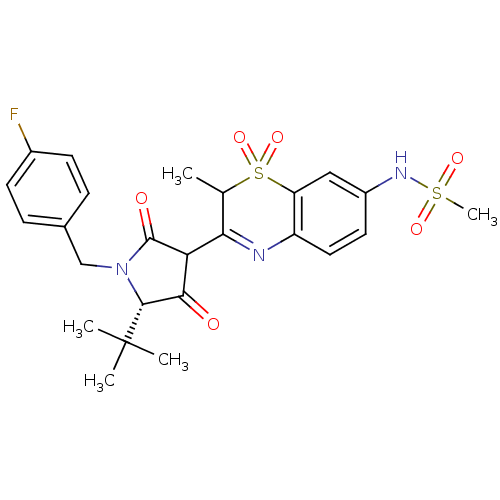
(CHEMBL584537 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...)Show SMILES CC1C(=Nc2ccc(NS(C)(=O)=O)cc2S1(=O)=O)C1C(=O)[C@@H](N(Cc2ccc(F)cc2)C1=O)C(C)(C)C |r,c:2| Show InChI InChI=1S/C25H28FN3O6S2/c1-14-21(27-18-11-10-17(28-36(5,32)33)12-19(18)37(14,34)35)20-22(30)23(25(2,3)4)29(24(20)31)13-15-6-8-16(26)9-7-15/h6-12,14,20,23,28H,13H2,1-5H3/t14?,20?,23-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5648-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.023
BindingDB Entry DOI: 10.7270/Q2T72HJM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data