 Found 6917 hits with Last Name = 'ali' and Initial = 'f'
Found 6917 hits with Last Name = 'ali' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM518
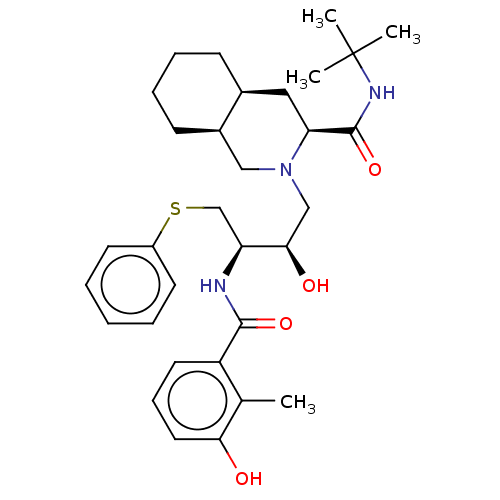
((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](CSc1ccccc1)NC(=O)c1cccc(O)c1C)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | -62.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9183
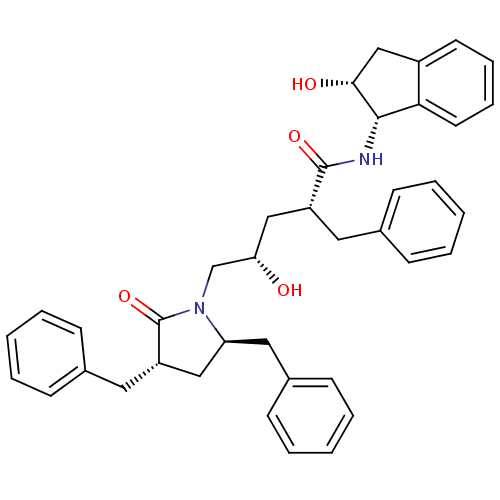
((2R,4S)-2-benzyl-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrr...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C39H42N2O4/c42-34(26-41-33(22-29-16-8-3-9-17-29)23-32(39(41)45)21-28-14-6-2-7-15-28)24-31(20-27-12-4-1-5-13-27)38(44)40-37-35-19-11-10-18-30(35)25-36(37)43/h1-19,31-34,36-37,42-43H,20-26H2,(H,40,44)/t31-,32+,33+,34+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Binding affinity to HIV-1 protease was determined |
Bioorg Med Chem Lett 8: 3631-6 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8Z5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131925
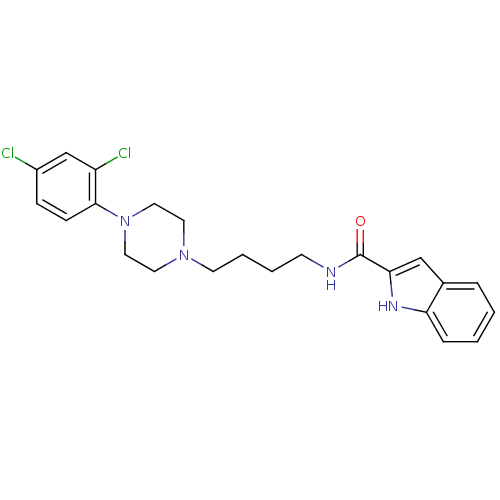
(1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4ccccc4[nH]3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H26Cl2N4O/c24-18-7-8-22(19(25)16-18)29-13-11-28(12-14-29)10-4-3-9-26-23(30)21-15-17-5-1-2-6-20(17)27-21/h1-2,5-8,15-16,27H,3-4,9-14H2,(H,26,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131930
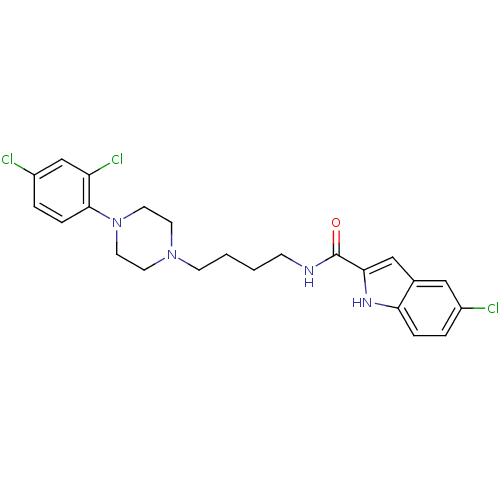
(5-Chloro-1H-indole-2-carboxylic acid {4-[4-(2,4-di...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4cc(Cl)ccc4[nH]3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H25Cl3N4O/c24-17-3-5-20-16(13-17)14-21(28-20)23(31)27-7-1-2-8-29-9-11-30(12-10-29)22-6-4-18(25)15-19(22)26/h3-6,13-15,28H,1-2,7-12H2,(H,27,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50003000
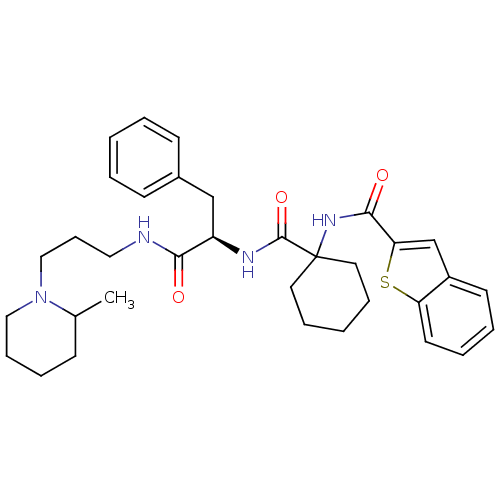
(CHEMBL394889)Show SMILES CC1CCCCN1CCCNC(=O)[C@@H](Cc1ccccc1)NC(=O)C1(CCCCC1)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C34H44N4O3S/c1-25-13-8-11-21-38(25)22-12-20-35-31(39)28(23-26-14-4-2-5-15-26)36-33(41)34(18-9-3-10-19-34)37-32(40)30-24-27-16-6-7-17-29(27)42-30/h2,4-7,14-17,24-25,28H,3,8-13,18-23H2,1H3,(H,35,39)(H,36,41)(H,37,40)/t25?,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131923
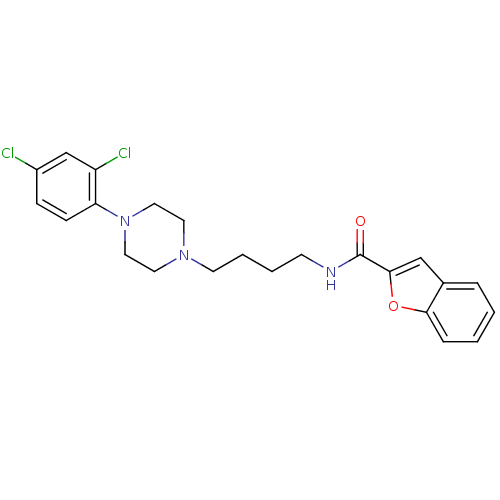
(Benzofuran-2-carboxylic acid {4-[4-(2,4-dichloro-p...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4ccccc4o3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H25Cl2N3O2/c24-18-7-8-20(19(25)16-18)28-13-11-27(12-14-28)10-4-3-9-26-23(29)22-15-17-5-1-2-6-21(17)30-22/h1-2,5-8,15-16H,3-4,9-14H2,(H,26,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131926
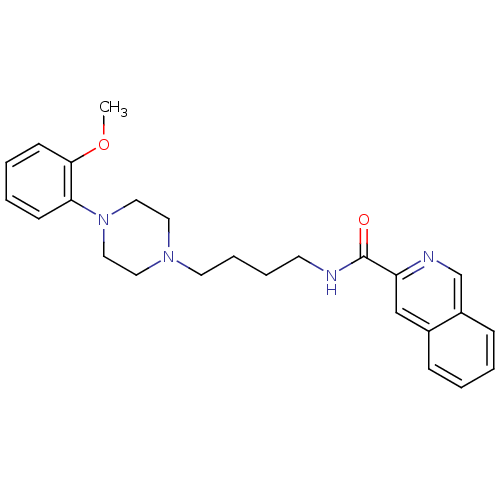
(CHEMBL127400 | CHEMBL129022 | Isoquinoline-3-carbo...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2cc3ccccc3cn2)CC1 Show InChI InChI=1S/C25H30N4O2/c1-31-24-11-5-4-10-23(24)29-16-14-28(15-17-29)13-7-6-12-26-25(30)22-18-20-8-2-3-9-21(20)19-27-22/h2-5,8-11,18-19H,6-7,12-17H2,1H3,(H,26,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305149
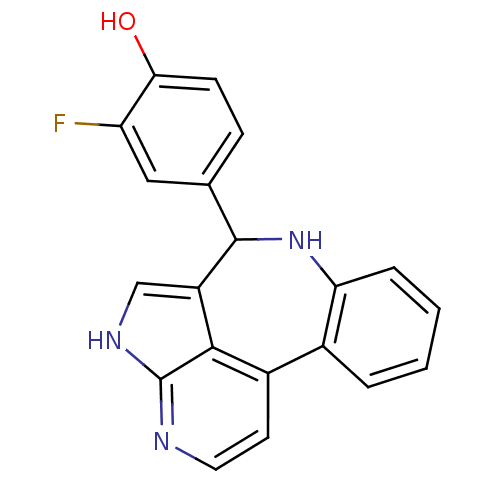
(2-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show InChI InChI=1S/C20H14FN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM9235
((2S)-N-(cyclopentylmethyl)-3-[(3S,5R)-3,5-dibenzyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C[C@@H](O)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O)CC1CCCC1 |r| Show InChI InChI=1S/C34H42N2O5S/c1-41-32-16-18-33(19-17-32)42(39,40)35(23-28-14-8-9-15-28)24-31(37)25-36-30(21-27-12-6-3-7-13-27)22-29(34(36)38)20-26-10-4-2-5-11-26/h2-7,10-13,16-19,28-31,37H,8-9,14-15,20-25H2,1H3/t29-,30-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Compound was assayed for inhibition against HIV protease activity |
Bioorg Med Chem Lett 8: 3637-42 (1999)
BindingDB Entry DOI: 10.7270/Q2GM86FH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9225
((2R,4S)-2-benzyl-5-[(3R,5R)-3,5-dibenzyl-2-oxopyrr...)Show SMILES [H][C@@]1(Cc2ccccc2)C[C@H](Cc2ccccc2)N(C[C@@H](O)C[C@@H](Cc2ccccc2)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)C1=O |r| Show InChI InChI=1S/C39H42N2O4/c42-34(26-41-33(22-29-16-8-3-9-17-29)23-32(39(41)45)21-28-14-6-2-7-15-28)24-31(20-27-12-4-1-5-13-27)38(44)40-37-35-19-11-10-18-30(35)25-36(37)43/h1-19,31-34,36-37,42-43H,20-26H2,(H,40,44)/t31-,32-,33+,34+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.5 | >-53.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Compound was assayed for inhibition against HIV protease activity |
Bioorg Med Chem Lett 8: 3637-42 (1999)
BindingDB Entry DOI: 10.7270/Q2GM86FH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305150
(3-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C20H14FN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002935
(CHEMBL242631 | MEN-14268)Show SMILES O=C(NCCCN1CCOCC1)[C@@H](Cc1ccccc1)NC(=O)C1(CCCC1)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C31H38N4O4S/c36-28(32-15-8-16-35-17-19-39-20-18-35)25(21-23-9-2-1-3-10-23)33-30(38)31(13-6-7-14-31)34-29(37)27-22-24-11-4-5-12-26(24)40-27/h1-5,9-12,22,25H,6-8,13-21H2,(H,32,36)(H,33,38)(H,34,37)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131921
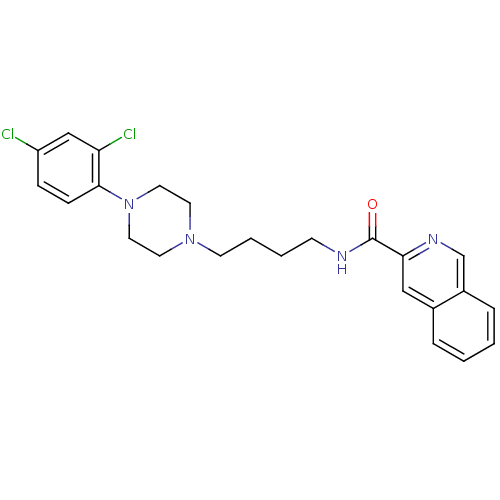
(CHEMBL340641 | Isoquinoline-3-carboxylic acid {4-[...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4ccccc4cn3)CC2)c(Cl)c1 Show InChI InChI=1S/C24H26Cl2N4O/c25-20-7-8-23(21(26)16-20)30-13-11-29(12-14-30)10-4-3-9-27-24(31)22-15-18-5-1-2-6-19(18)17-28-22/h1-2,5-8,15-17H,3-4,9-14H2,(H,27,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002941

(CHEMBL428203)Show SMILES O=C(NCCCN1CCOCC1)[C@@H](Cc1ccccc1)NC(=O)C1(CCCCC1)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C32H40N4O4S/c37-29(33-16-9-17-36-18-20-40-21-19-36)26(22-24-10-3-1-4-11-24)34-31(39)32(14-7-2-8-15-32)35-30(38)28-23-25-12-5-6-13-27(25)41-28/h1,3-6,10-13,23,26H,2,7-9,14-22H2,(H,33,37)(H,34,39)(H,35,38)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305157
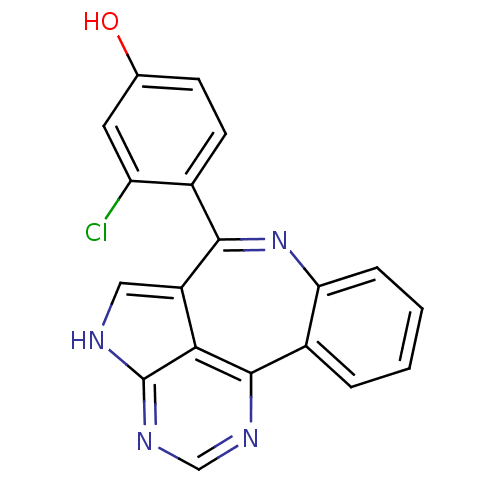
(3-chloro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(c(Cl)c1)-c1nc2ccccc2c2ncnc3[nH]cc1c23 Show InChI InChI=1S/C19H11ClN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9229
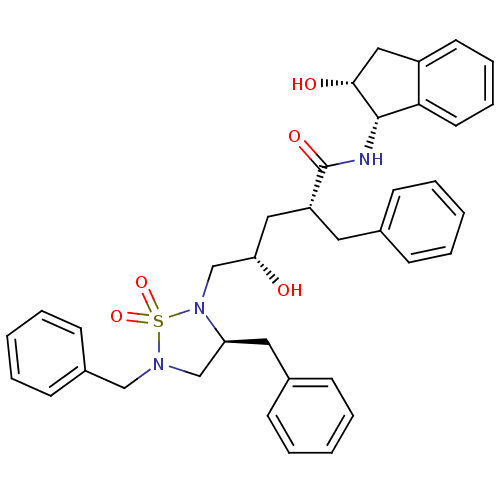
((2R,4S)-2-benzyl-5-[(3S)-3,5-dibenzyl-1,1-dioxo-1,...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)CN(Cc2ccccc2)S1(=O)=O |r| Show InChI InChI=1S/C37H41N3O5S/c41-33(22-31(20-27-12-4-1-5-13-27)37(43)38-36-34-19-11-10-18-30(34)23-35(36)42)26-40-32(21-28-14-6-2-7-15-28)25-39(46(40,44)45)24-29-16-8-3-9-17-29/h1-19,31-33,35-36,41-42H,20-26H2,(H,38,43)/t31-,32+,33+,35-,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131920
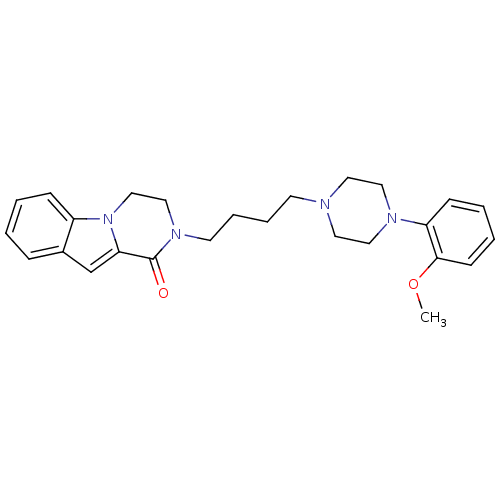
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2CCn3c(cc4ccccc34)C2=O)CC1 Show InChI InChI=1S/C26H32N4O2/c1-32-25-11-5-4-10-23(25)28-16-14-27(15-17-28)12-6-7-13-29-18-19-30-22-9-3-2-8-21(22)20-24(30)26(29)31/h2-5,8-11,20H,6-7,12-19H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 4
(Homo sapiens (Human)) | BDBM50226415
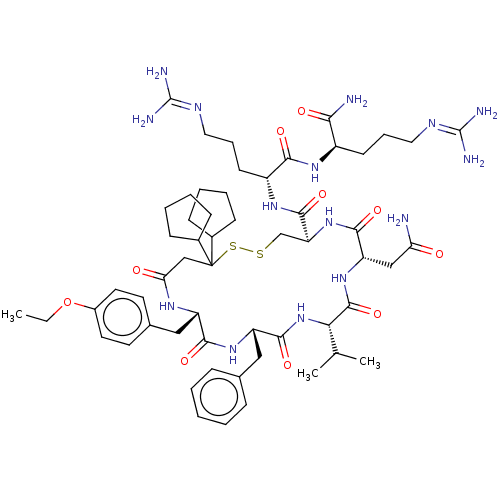
(CHEMBL3142312)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C([#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)([#6]-2-[#6]-[#6]-[#6]-[#6]-2)[#6]-2-[#6]-[#6]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C57H87N15O10S2/c1-4-82-38-24-22-35(23-25-38)29-41-50(77)69-42(28-34-14-6-5-7-15-34)52(79)72-47(33(2)3)54(81)70-43(30-45(58)73)51(78)71-44(32-83-84-57(31-46(74)66-41,36-16-8-9-17-36)37-18-10-11-19-37)53(80)68-40(21-13-27-65-56(62)63)49(76)67-39(48(59)75)20-12-26-64-55(60)61/h5-7,14-15,22-25,33,36-37,39-44,47H,4,8-13,16-21,26-32H2,1-3H3,(H2,58,73)(H2,59,75)(H,66,74)(H,67,76)(H,68,80)(H,69,77)(H,70,81)(H,71,78)(H,72,79)(H4,60,61,64)(H4,62,63,65)/t39-,40-,41+,42+,43+,44+,47+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2291-4 (1987)
BindingDB Entry DOI: 10.7270/Q2MG7RRN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50119380
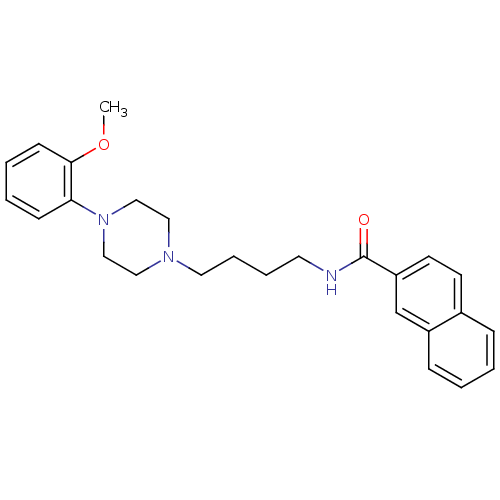
(CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C26H31N3O2/c1-31-25-11-5-4-10-24(25)29-18-16-28(17-19-29)15-7-6-14-27-26(30)23-13-12-21-8-2-3-9-22(21)20-23/h2-5,8-13,20H,6-7,14-19H2,1H3,(H,27,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. |
J Med Chem 46: 3822-39 (2003)
Article DOI: 10.1021/jm0211220
BindingDB Entry DOI: 10.7270/Q2H132RF |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288109
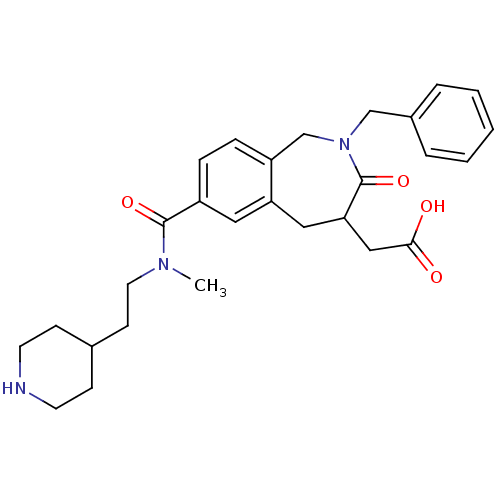
(CHEMBL82123 | {2-Benzyl-7-[methyl-(2-piperidin-4-y...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(Cc3ccccc3)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C28H35N3O4/c1-30(14-11-20-9-12-29-13-10-20)27(34)22-7-8-23-19-31(18-21-5-3-2-4-6-21)28(35)25(17-26(32)33)16-24(23)15-22/h2-8,15,20,25,29H,9-14,16-19H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50294132
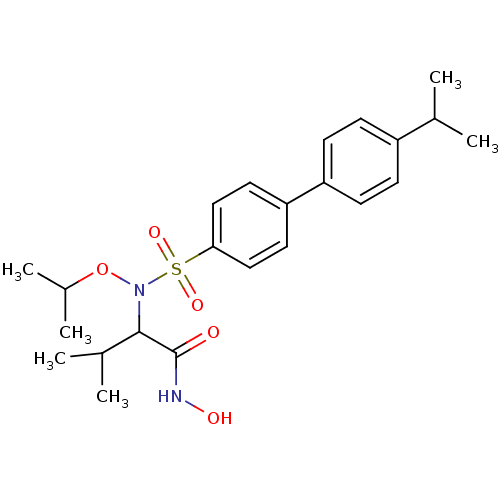
(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305155
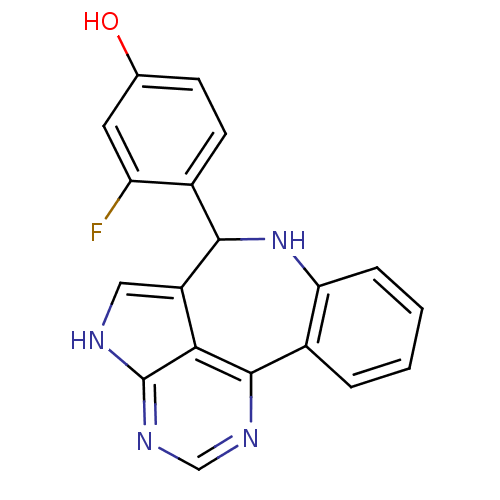
(3-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ncnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C19H13FN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002943
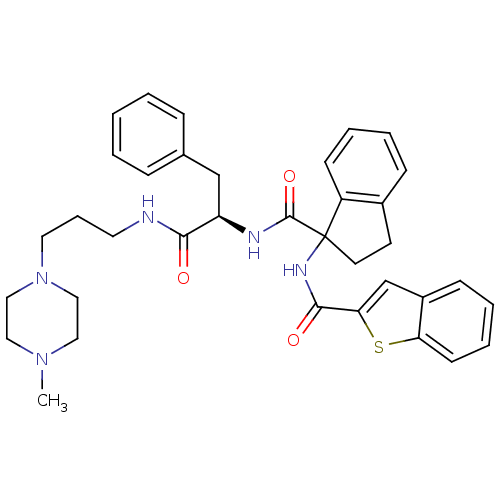
(CHEMBL394878)Show SMILES CN1CCN(CCCNC(=O)[C@@H](Cc2ccccc2)NC(=O)C2(CCc3ccccc23)NC(=O)c2cc3ccccc3s2)CC1 Show InChI InChI=1S/C36H41N5O3S/c1-40-20-22-41(23-21-40)19-9-18-37-33(42)30(24-26-10-3-2-4-11-26)38-35(44)36(17-16-27-12-5-7-14-29(27)36)39-34(43)32-25-28-13-6-8-15-31(28)45-32/h2-8,10-15,25,30H,9,16-24H2,1H3,(H,37,42)(H,38,44)(H,39,43)/t30-,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305156
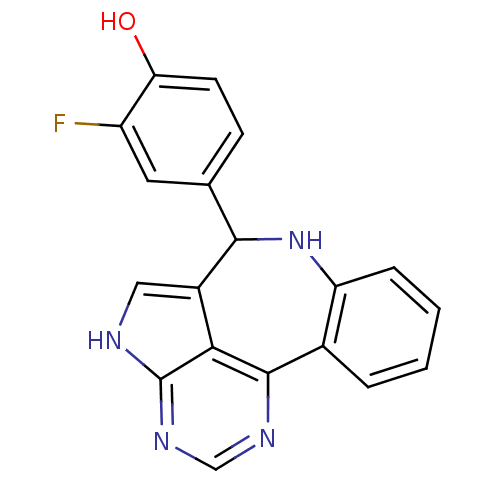
(2-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show InChI InChI=1S/C19H13FN4O/c20-13-7-10(5-6-15(13)25)17-12-8-21-19-16(12)18(22-9-23-19)11-3-1-2-4-14(11)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002943

(CHEMBL394878)Show SMILES CN1CCN(CCCNC(=O)[C@@H](Cc2ccccc2)NC(=O)C2(CCc3ccccc23)NC(=O)c2cc3ccccc3s2)CC1 Show InChI InChI=1S/C36H41N5O3S/c1-40-20-22-41(23-21-40)19-9-18-37-33(42)30(24-26-10-3-2-4-11-26)38-35(44)36(17-16-27-12-5-7-14-29(27)36)39-34(43)32-25-28-13-6-8-15-31(28)45-32/h2-8,10-15,25,30H,9,16-24H2,1H3,(H,37,42)(H,38,44)(H,39,43)/t30-,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052204
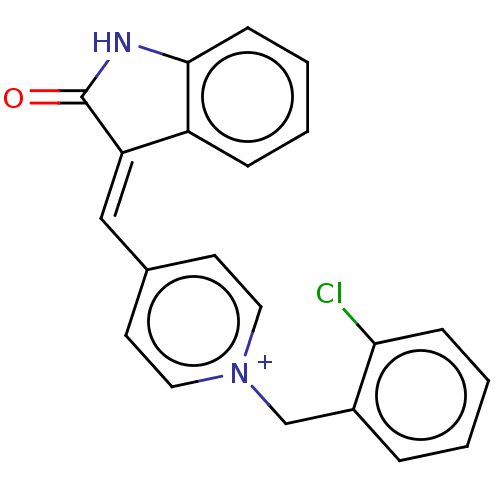
(CHEMBL3318392)Show SMILES [Cl-].Clc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15ClN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50027905
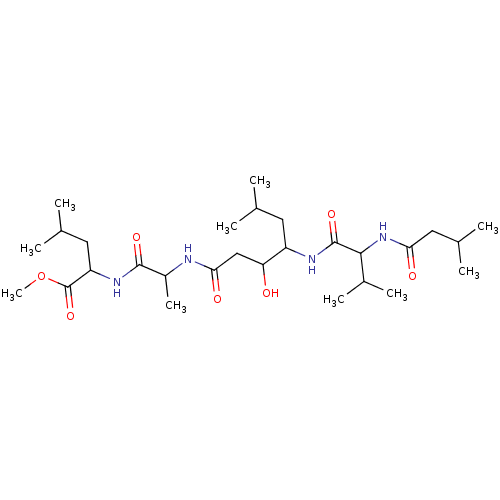
(3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...)Show SMILES COC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)CC(O)C(CC(C)C)NC(=O)C(NC(=O)CC(C)C)C(C)C Show InChI InChI=1S/C28H52N4O7/c1-15(2)11-20(30-27(37)25(18(7)8)32-23(34)13-17(5)6)22(33)14-24(35)29-19(9)26(36)31-21(12-16(3)4)28(38)39-10/h15-22,25,33H,11-14H2,1-10H3,(H,29,35)(H,30,37)(H,31,36)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for time dependent inhibition constant against porcine pepsin |
J Med Chem 26: 904-10 (1983)
BindingDB Entry DOI: 10.7270/Q2057DX2 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9230
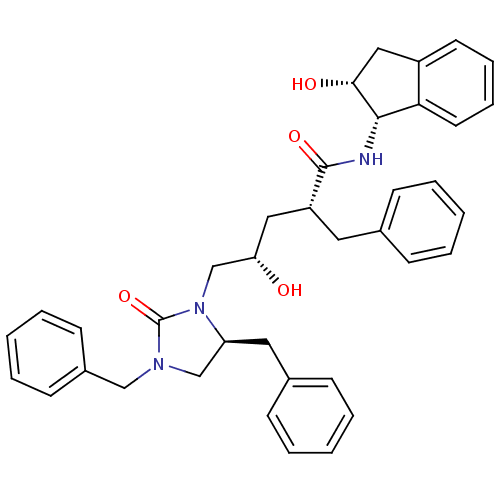
((2R,4S)-2-benzyl-5-[(5S)-3,5-dibenzyl-2-oxoimidazo...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)CN(Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C38H41N3O4/c42-33(26-41-32(21-28-14-6-2-7-15-28)25-40(38(41)45)24-29-16-8-3-9-17-29)22-31(20-27-12-4-1-5-13-27)37(44)39-36-34-19-11-10-18-30(34)23-35(36)43/h1-19,31-33,35-36,42-43H,20-26H2,(H,39,44)/t31-,32+,33+,35-,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 10: 1159-62 (2000)
Article DOI: 10.1016/s0960-894x(00)00163-3
BindingDB Entry DOI: 10.7270/Q2X63K5G |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020671

(13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C46H69N11O8S2/c1-4-65-31-17-15-30(16-18-31)25-34-41(61)55-35(24-29-12-7-5-8-13-29)42(62)57-38(28(2)3)44(64)54-33(19-22-47)40(60)56-36(43(63)53-32(39(48)59)14-11-23-51-45(49)50)27-66-67-46(26-37(58)52-34)20-9-6-10-21-46/h5,7-8,12-13,15-18,28,32-36,38H,4,6,9-11,14,19-27,47H2,1-3H3,(H2,48,59)(H,52,58)(H,53,63)(H,54,64)(H,55,61)(H,56,60)(H,57,62)(H4,49,50,51)/t32-,33-,34+,35-,36-,38+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288120

(CHEMBL313768 | {2-Cyclohexyl-7-[methyl-(2-piperidi...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(C3CCCCC3)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C27H39N3O4/c1-29(14-11-19-9-12-28-13-10-19)26(33)20-7-8-21-18-30(24-5-3-2-4-6-24)27(34)23(17-25(31)32)16-22(21)15-20/h7-8,15,19,23-24,28H,2-6,9-14,16-18H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002936
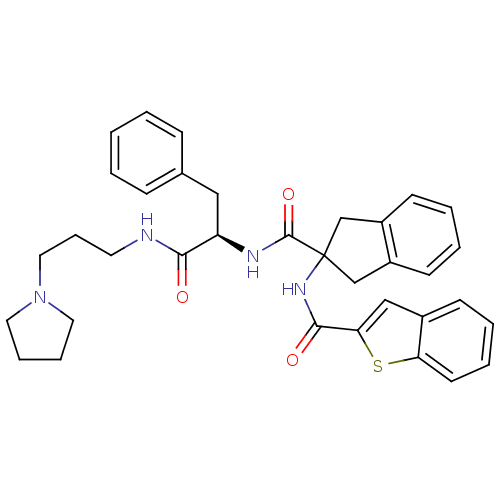
(CHEMBL394420)Show SMILES O=C(NCCCN1CCCC1)[C@@H](Cc1ccccc1)NC(=O)C1(Cc2ccccc2C1)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C35H38N4O3S/c40-32(36-17-10-20-39-18-8-9-19-39)29(21-25-11-2-1-3-12-25)37-34(42)35(23-27-14-4-5-15-28(27)24-35)38-33(41)31-22-26-13-6-7-16-30(26)43-31/h1-7,11-16,22,29H,8-10,17-21,23-24H2,(H,36,40)(H,37,42)(H,38,41)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002946
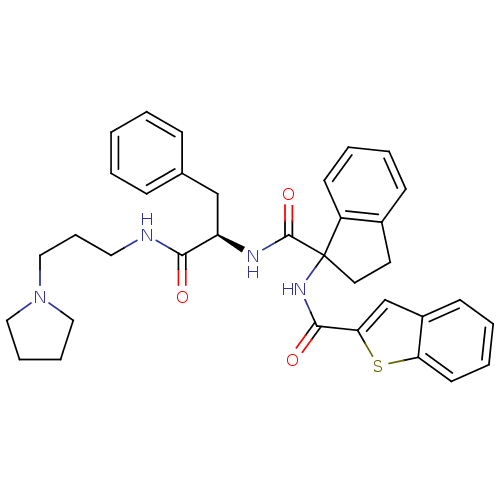
(CHEMBL394418)Show SMILES O=C(NCCCN1CCCC1)[C@@H](Cc1ccccc1)NC(=O)C1(CCc2ccccc12)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C35H38N4O3S/c40-32(36-19-10-22-39-20-8-9-21-39)29(23-25-11-2-1-3-12-25)37-34(42)35(18-17-26-13-4-6-15-28(26)35)38-33(41)31-24-27-14-5-7-16-30(27)43-31/h1-7,11-16,24,29H,8-10,17-23H2,(H,36,40)(H,37,42)(H,38,41)/t29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002940
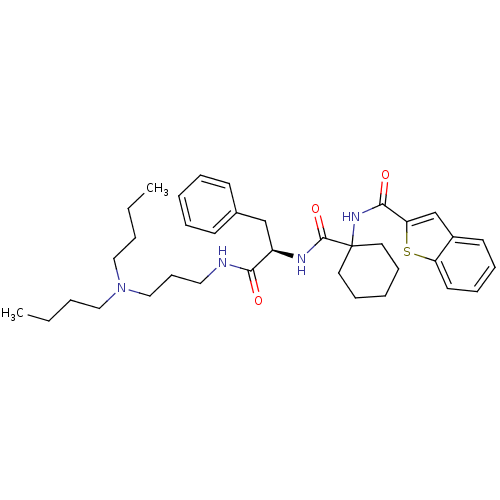
(CHEMBL237097)Show SMILES CCCCN(CCCC)CCCNC(=O)[C@@H](Cc1ccccc1)NC(=O)C1(CCCCC1)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C36H50N4O3S/c1-3-5-23-40(24-6-4-2)25-15-22-37-33(41)30(26-28-16-9-7-10-17-28)38-35(43)36(20-13-8-14-21-36)39-34(42)32-27-29-18-11-12-19-31(29)44-32/h7,9-12,16-19,27,30H,3-6,8,13-15,20-26H2,1-2H3,(H,37,41)(H,38,43)(H,39,42)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50002946

(CHEMBL394418)Show SMILES O=C(NCCCN1CCCC1)[C@@H](Cc1ccccc1)NC(=O)C1(CCc2ccccc12)NC(=O)c1cc2ccccc2s1 Show InChI InChI=1S/C35H38N4O3S/c40-32(36-19-10-22-39-20-8-9-21-39)29(23-25-11-2-1-3-12-25)37-34(42)35(18-17-26-13-4-6-15-28(26)35)38-33(41)31-24-27-14-5-7-16-30(27)43-31/h1-7,11-16,24,29H,8-10,17-23H2,(H,36,40)(H,37,42)(H,38,41)/t29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche SpA
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 17: 4841-4 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.06.053
BindingDB Entry DOI: 10.7270/Q22F7PXR |
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Homo sapiens (Human)) | BDBM50541929

(CHEMBL1738785)Show SMILES Cn1cnc2n(C)c(=O)n(CCCn3c(=O)n(C)c4[nH]cnc4c3=O)c(=O)c12 Show InChI InChI=1S/C16H18N8O4/c1-20-8-19-12-10(20)14(26)24(16(28)22(12)3)6-4-5-23-13(25)9-11(18-7-17-9)21(2)15(23)27/h7-8H,4-6H2,1-3H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288118
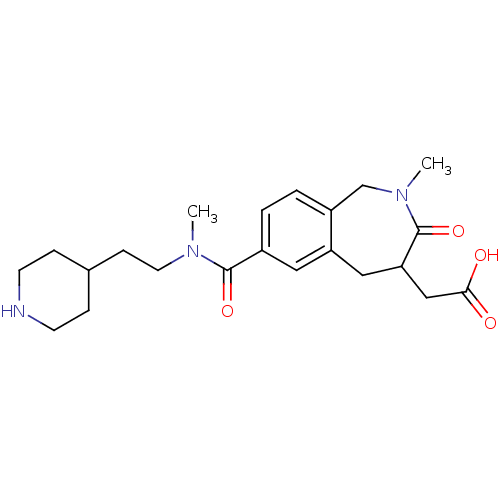
(CHEMBL312122 | {2-Methyl-7-[methyl-(2-piperidin-4-...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(C)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C22H31N3O4/c1-24(10-7-15-5-8-23-9-6-15)21(28)16-3-4-17-14-25(2)22(29)19(13-20(26)27)12-18(17)11-16/h3-4,11,15,19,23H,5-10,12-14H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50074035
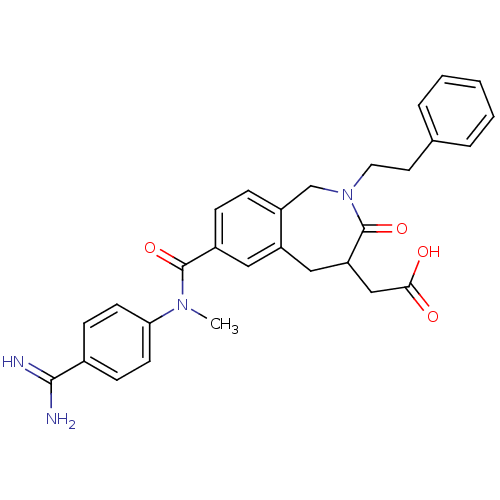
(CHEMBL82980 | {7-[(4-Carbamimidoyl-phenyl)-methyl-...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Cc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H30N4O4/c1-32(25-11-9-20(10-12-25)27(30)31)28(36)21-7-8-22-18-33(14-13-19-5-3-2-4-6-19)29(37)24(17-26(34)35)16-23(22)15-21/h2-12,15,24H,13-14,16-18H2,1H3,(H3,30,31)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50054826
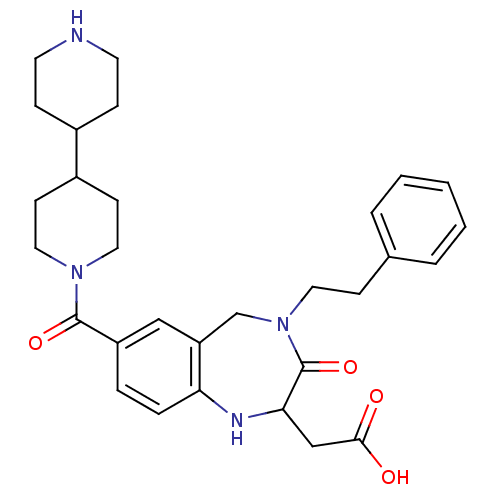
(CHEMBL144474 | [7-([4,4']Bipiperidinyl-1-carbonyl)...)Show SMILES OC(=O)CC1Nc2ccc(cc2CN(CCc2ccccc2)C1=O)C(=O)N1CCC(CC1)C1CCNCC1 Show InChI InChI=1S/C30H38N4O4/c35-28(36)19-27-30(38)34(15-10-21-4-2-1-3-5-21)20-25-18-24(6-7-26(25)32-27)29(37)33-16-11-23(12-17-33)22-8-13-31-14-9-22/h1-7,18,22-23,27,31-32H,8-17,19-20H2,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of binding to purified integrin alphaIIb-beta3 of human platelets |
J Med Chem 39: 4867-70 (1997)
Article DOI: 10.1021/jm960558a
BindingDB Entry DOI: 10.7270/Q2GH9H16 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50294132

(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP13-mediated collagen degradation by SDS-PAGE |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50027909
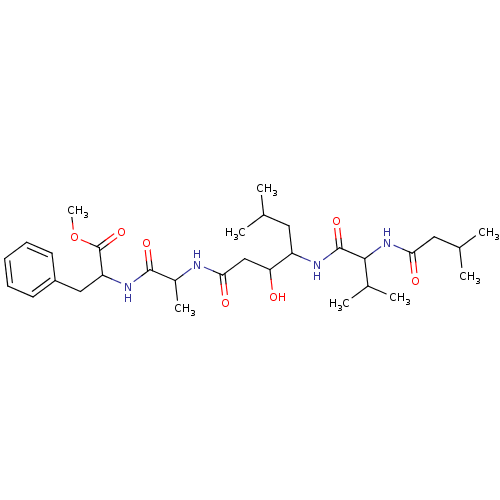
(2-(2-{3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-b...)Show SMILES COC(=O)C(Cc1ccccc1)NC(=O)C(C)NC(=O)CC(O)C(CC(C)C)NC(=O)C(NC(=O)CC(C)C)C(C)C Show InChI InChI=1S/C31H50N4O7/c1-18(2)14-23(33-30(40)28(20(5)6)35-26(37)15-19(3)4)25(36)17-27(38)32-21(7)29(39)34-24(31(41)42-8)16-22-12-10-9-11-13-22/h9-13,18-21,23-25,28,36H,14-17H2,1-8H3,(H,32,38)(H,33,40)(H,34,39)(H,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for time dependent inhibition constant against porcine pepsin |
J Med Chem 26: 904-10 (1983)
BindingDB Entry DOI: 10.7270/Q2057DX2 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288115
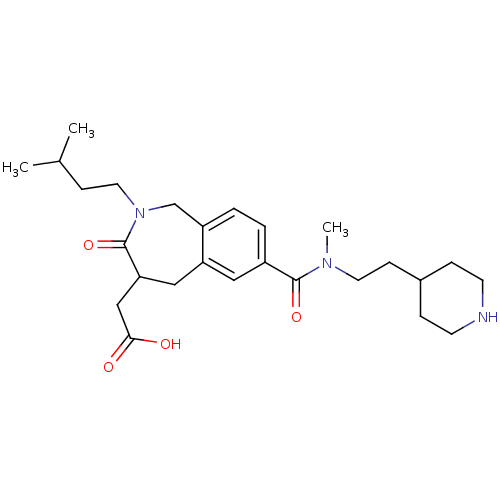
(CHEMBL82746 | {2-(3-Methyl-butyl)-7-[methyl-(2-pip...)Show SMILES CC(C)CCN1Cc2ccc(cc2CC(CC(O)=O)C1=O)C(=O)N(C)CCC1CCNCC1 Show InChI InChI=1S/C26H39N3O4/c1-18(2)8-13-29-17-21-5-4-20(14-22(21)15-23(26(29)33)16-24(30)31)25(32)28(3)12-9-19-6-10-27-11-7-19/h4-5,14,18-19,23,27H,6-13,15-17H2,1-3H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data