

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaounde Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad... | J Nat Prod 74: 1370-8 (2011) Article DOI: 10.1021/np100896w BindingDB Entry DOI: 10.7270/Q2WH2QC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93134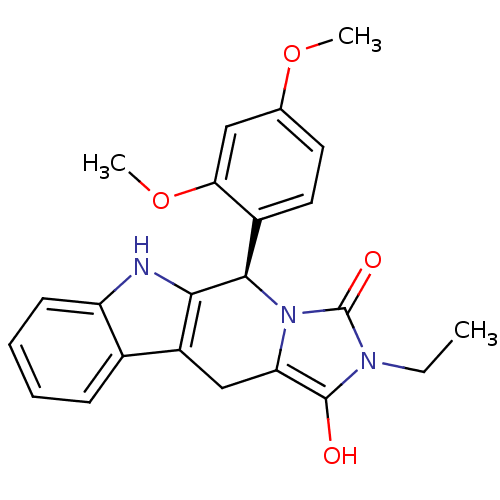 (PDE5 Inhibitor, 5 | PDE5 Inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93134 (PDE5 Inhibitor, 5 | PDE5 Inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93140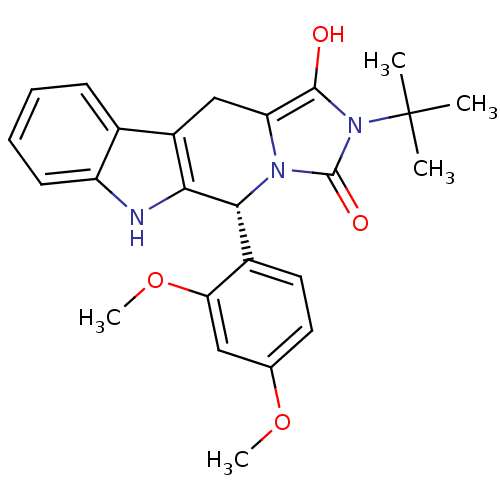 (PDE5 Inhibitor, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93141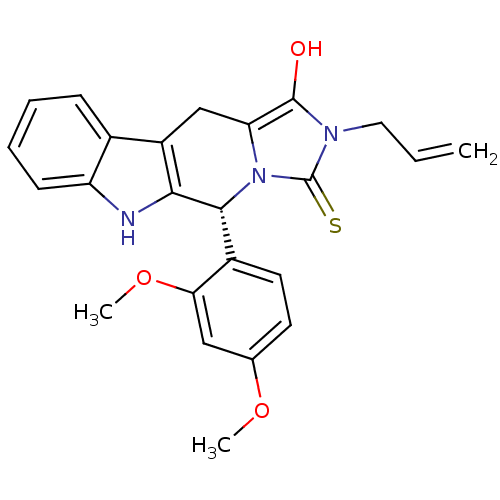 (PDE5 Inhibitor, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93136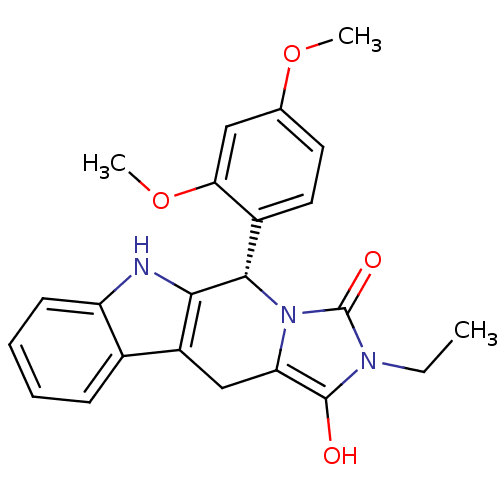 (PDE5 Inhibitor, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM50239965 (Bromhexine | CHEBI:77032) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420291 (0591-5329 | 4-(Methoxycarbonyl)phenyl thiophene-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM50022172 ((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization measured at 1 min interval for 1 hr by fluorescence plate reader assay | Bioorg Med Chem Lett 26: 4527-4535 (2016) Article DOI: 10.1016/j.bmcl.2016.06.044 BindingDB Entry DOI: 10.7270/Q2RF5ZJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50536788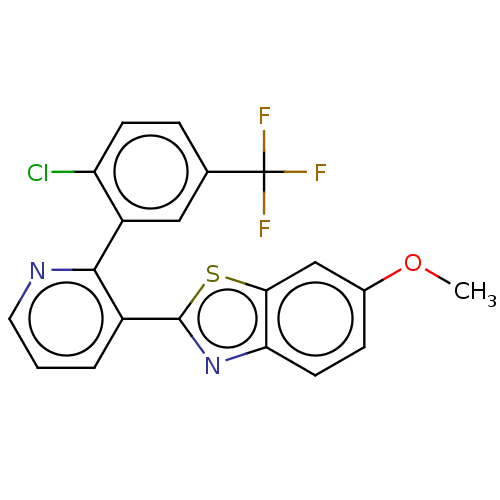 (CHEMBL4537424) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization measured at 1 min interval for 1 hr by fluorescence plate reader assay | Bioorg Med Chem Lett 26: 4527-4535 (2016) Article DOI: 10.1016/j.bmcl.2016.06.044 BindingDB Entry DOI: 10.7270/Q2RF5ZJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50536789 (CHEMBL4549647) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization measured at 1 min interval for 1 hr by fluorescence plate reader assay | Bioorg Med Chem Lett 26: 4527-4535 (2016) Article DOI: 10.1016/j.bmcl.2016.06.044 BindingDB Entry DOI: 10.7270/Q2RF5ZJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93137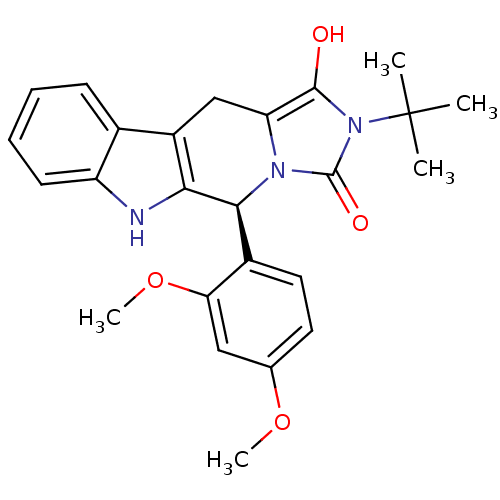 (PDE5 Inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93135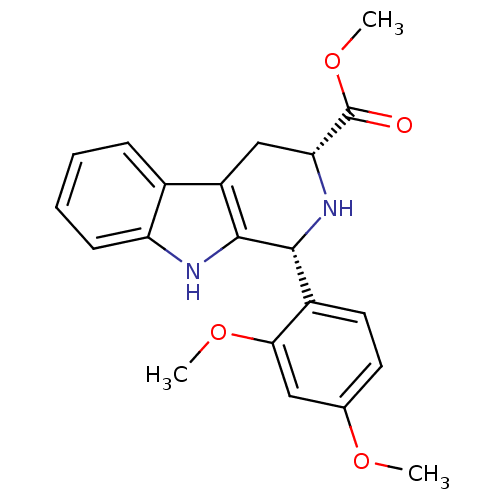 (PDE5 Inhibitor, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420294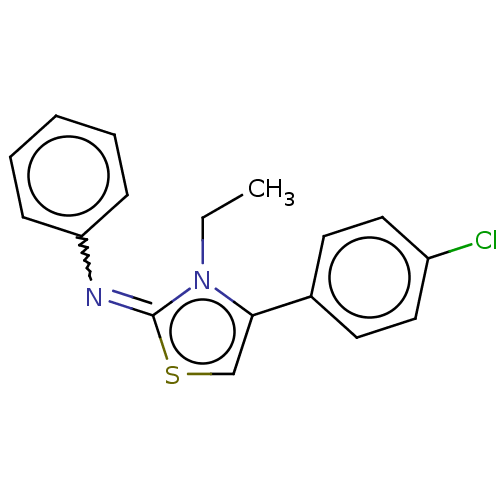 (8008-1235 | N-[4-(4-chlorophenyl)-3-ethyl-1,3-thia...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420292 (4401-0077 | Antidepressant agent 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE UniChem | Article PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM93138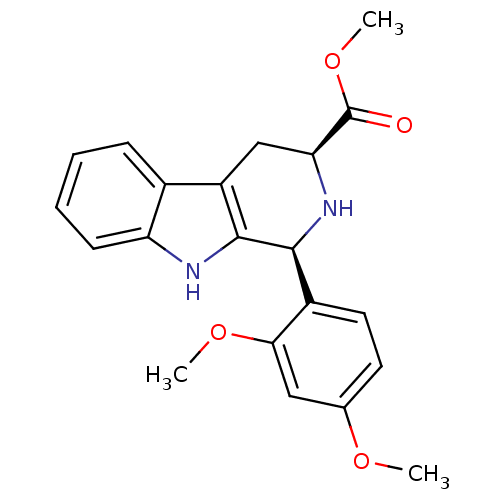 (PDE5 Inhibitor, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
German University in Cairo | Assay Description PDE activity was measured using a modification of the IMAP fluorescence polarization phosphodiesterase assay from Molecular Devices. The assay was m... | International Journal of Medicinal Chemistry 2011: 1-9 (2011) BindingDB Entry DOI: 10.7270/Q21R6P59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50349974 (CHEMBL1812485) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaounde Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad... | J Nat Prod 74: 1370-8 (2011) Article DOI: 10.1021/np100896w BindingDB Entry DOI: 10.7270/Q2WH2QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50240896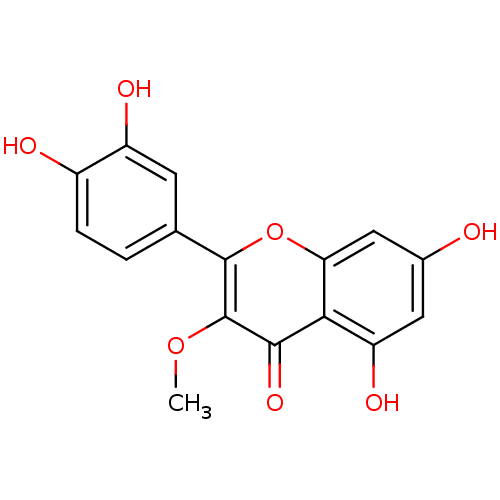 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-methoxy-4H...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaounde Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad... | J Nat Prod 74: 1370-8 (2011) Article DOI: 10.1021/np100896w BindingDB Entry DOI: 10.7270/Q2WH2QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50349976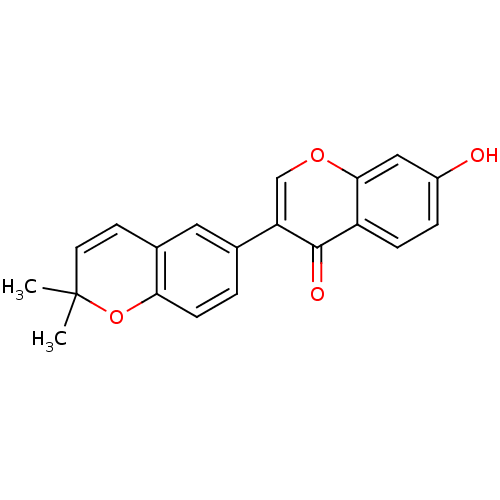 (ISOWIGHTEONE) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaounde Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad... | J Nat Prod 74: 1370-8 (2011) Article DOI: 10.1021/np100896w BindingDB Entry DOI: 10.7270/Q2WH2QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50349975 (CHEMBL1812593) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaounde Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate ad... | J Nat Prod 74: 1370-8 (2011) Article DOI: 10.1021/np100896w BindingDB Entry DOI: 10.7270/Q2WH2QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||