

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133817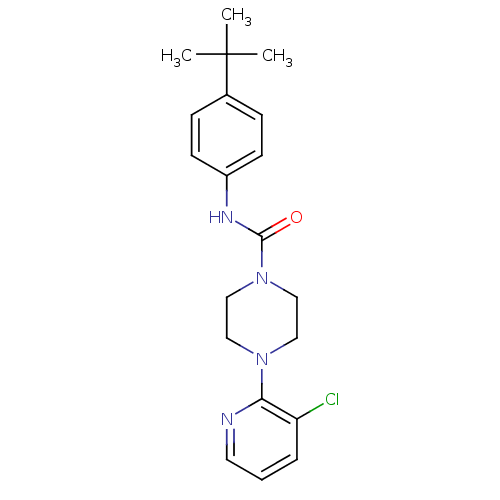 (4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pudue Pharma Discovery Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 377-86 (2003) Article DOI: 10.1124/jpet.102.045674 BindingDB Entry DOI: 10.7270/Q2TX3CX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186527 ((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523342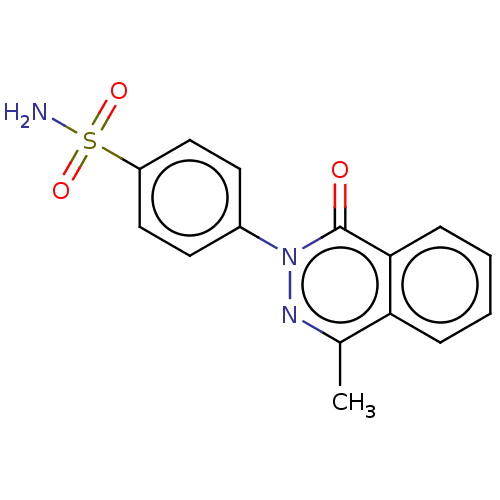 (CHEMBL4527427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50370598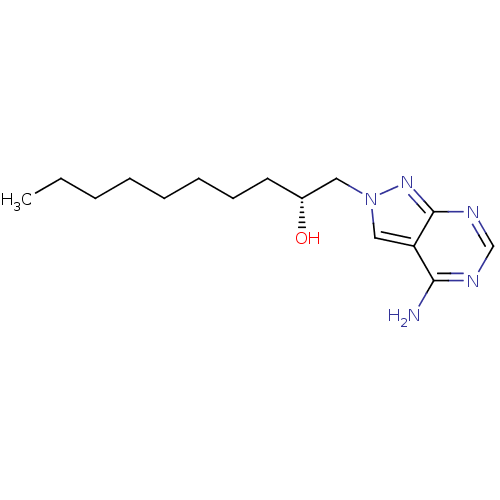 (CHEMBL1651379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibitory constant against bovine spleen Adenosine deaminase | J Med Chem 48: 5162-74 (2005) Article DOI: 10.1021/jm050136d BindingDB Entry DOI: 10.7270/Q2542PCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523332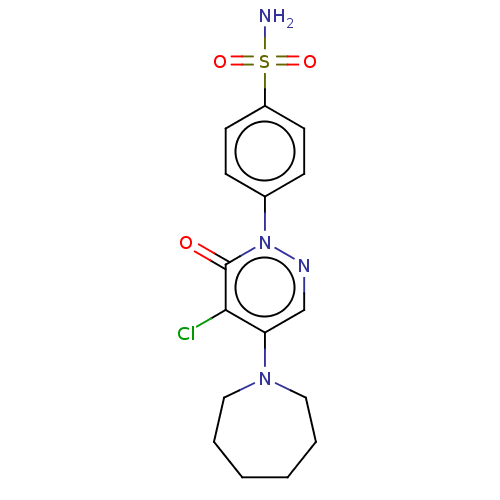 (CHEMBL4569531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523339 (CHEMBL4542010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523348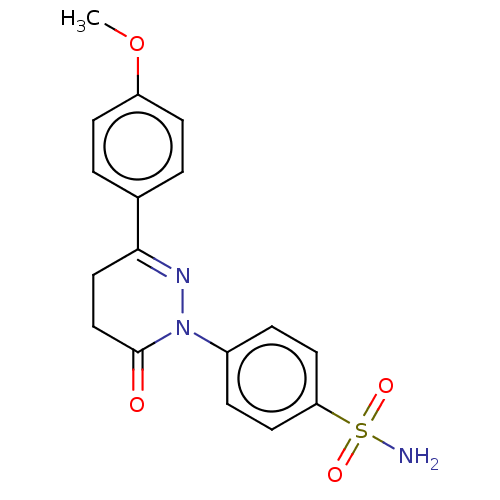 (CHEMBL4519564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523344 (CHEMBL4566532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536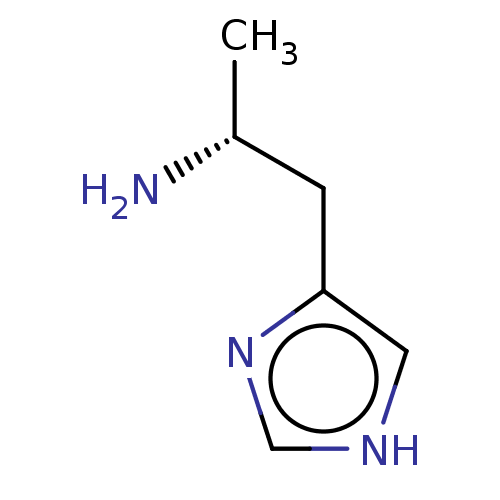 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50531530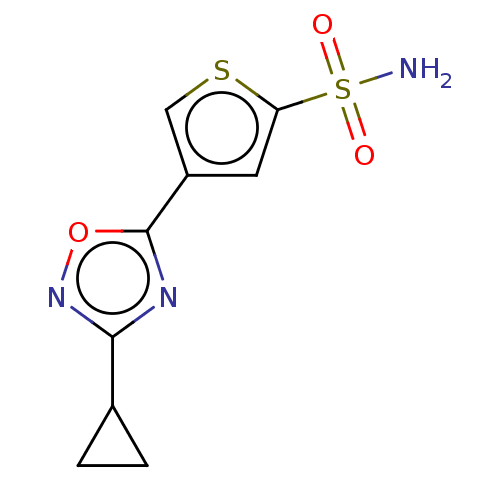 (CHEMBL4590200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 assessed as reduction in CO2 hydration after 15 mins by phenol red staining-based stopped flow assay | Eur J Med Chem 164: 92-105 (2019) Article DOI: 10.1016/j.ejmech.2018.12.049 BindingDB Entry DOI: 10.7270/Q2R78JPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523338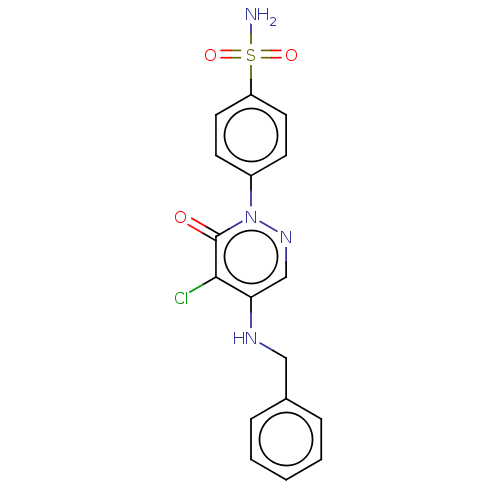 (CHEMBL4563416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186522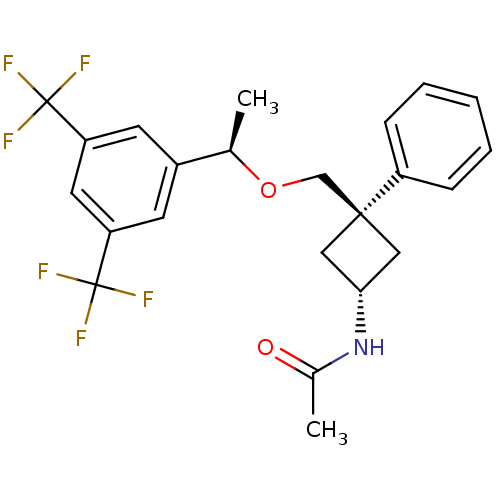 (CHEMBL379072 | trans-N-{3-[(R)-1-(3,5-bis-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411339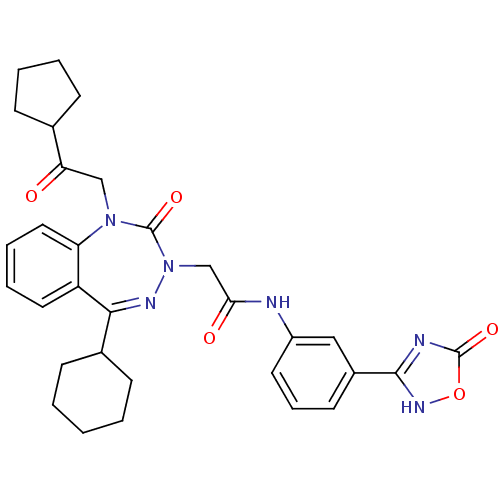 (CHEMBL227276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629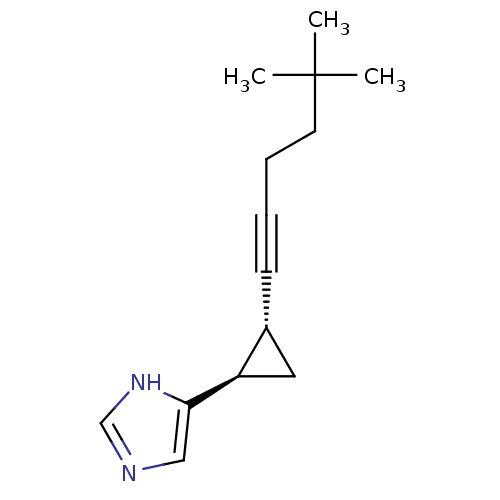 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50531505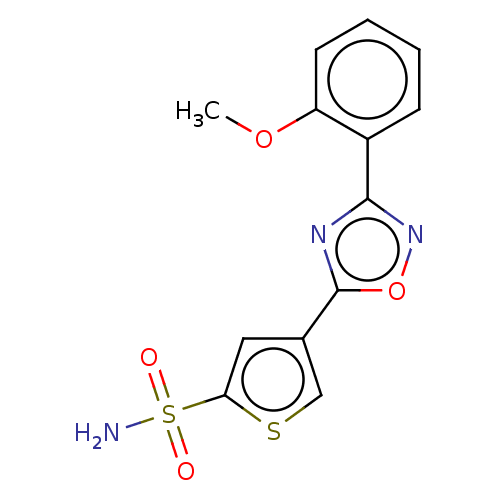 (CHEMBL4560251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 assessed as reduction in CO2 hydration after 15 mins by phenol red staining-based stopped flow assay | Eur J Med Chem 164: 92-105 (2019) Article DOI: 10.1016/j.ejmech.2018.12.049 BindingDB Entry DOI: 10.7270/Q2R78JPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50171394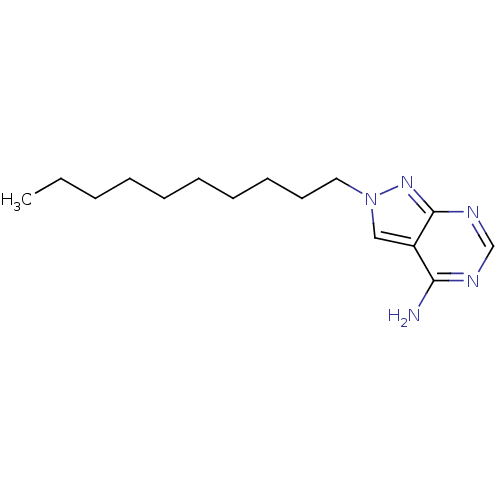 (2-Decyl-2H-pyrazolo[3,4-d]pyrimidin-4-ylamine | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibitory constant against bovine spleen Adenosine deaminase | J Med Chem 48: 5162-74 (2005) Article DOI: 10.1021/jm050136d BindingDB Entry DOI: 10.7270/Q2542PCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50056102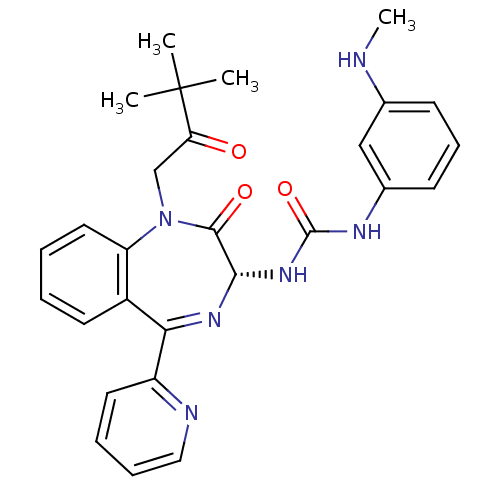 ((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328970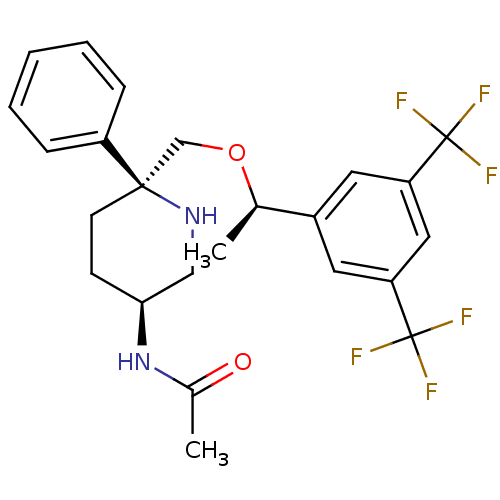 (CHEMBL1270066 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253857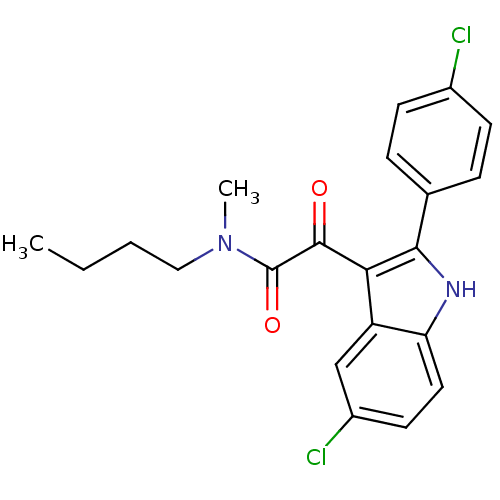 (CHEMBL460998 | N-butyl-2-(5-chloro-2-(4-chlorophen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig ileum LMMP homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253893 (2-(5-chloro-2-(4-chlorophenyl)-1H-indol-3-yl)-N-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50318267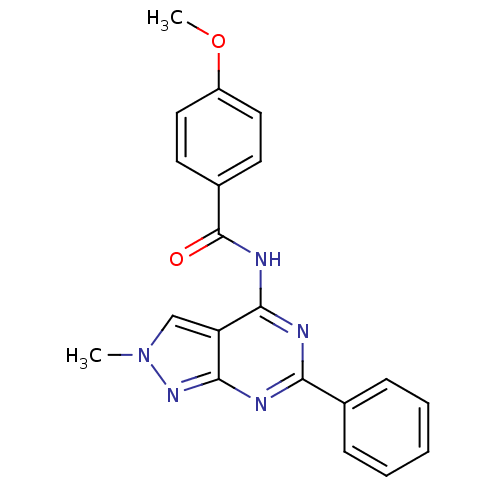 (4-methoxy-N-(2-methyl-6-phenyl-2H-pyrazolo[3,4-d]p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 53: 3954-63 (2010) Article DOI: 10.1021/jm901785w BindingDB Entry DOI: 10.7270/Q2N017HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328981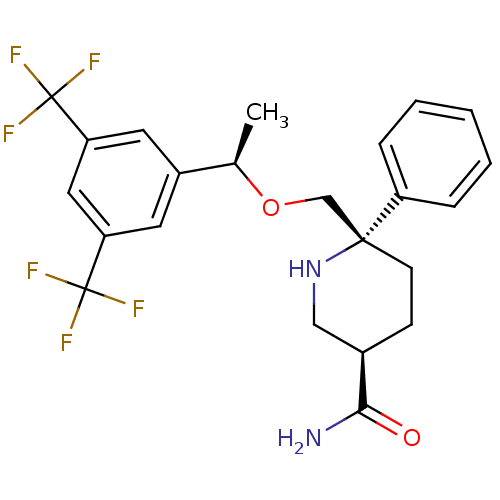 ((3R,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116745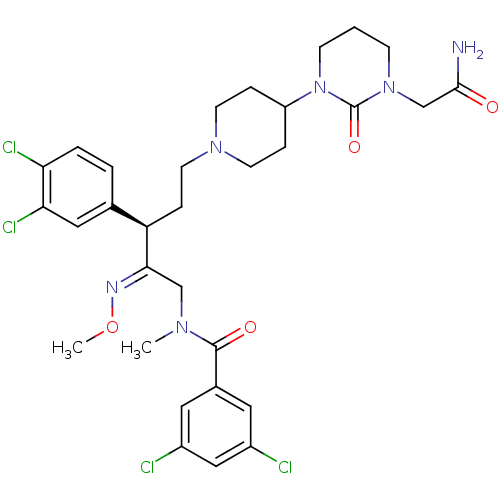 (CHEMBL78284 | N-{(R)-5-[4-(3-Carbamoylmethyl-2-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 2 in CHO cells using [3H]-NKA as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]Cl-DPDPE from human recombinant delta opioid receptor expressed in CHO cells | J Med Chem 47: 6645-8 (2004) Article DOI: 10.1021/jm040817t BindingDB Entry DOI: 10.7270/Q2C53MNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243425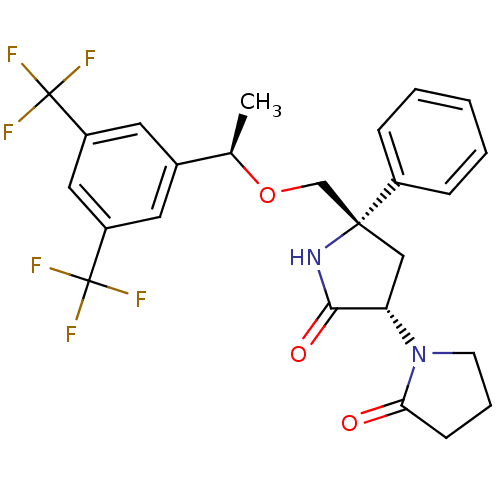 (1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243428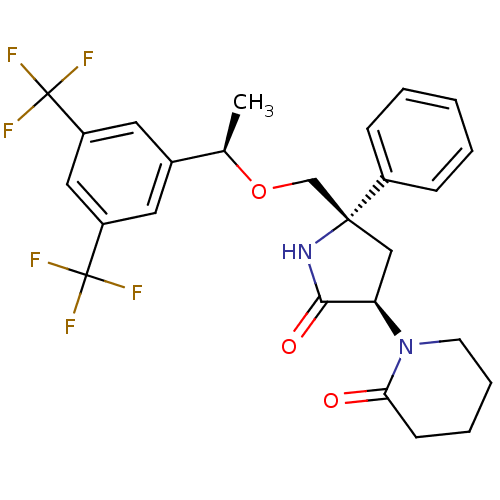 (1-((3R,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243427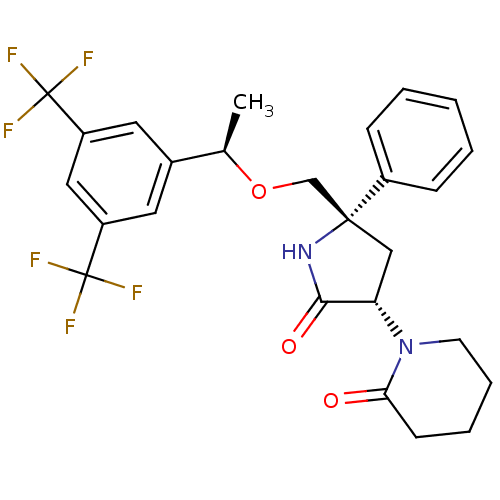 (1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411341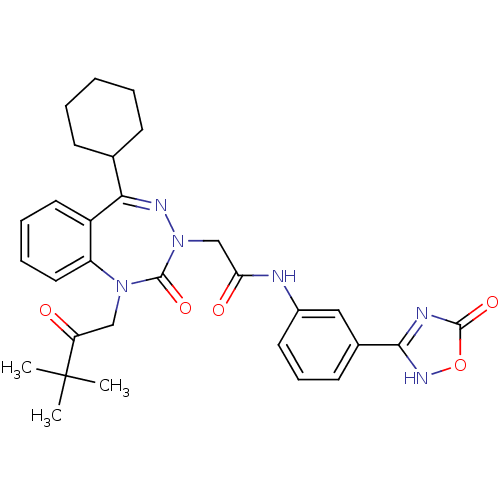 (CHEMBL226583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198126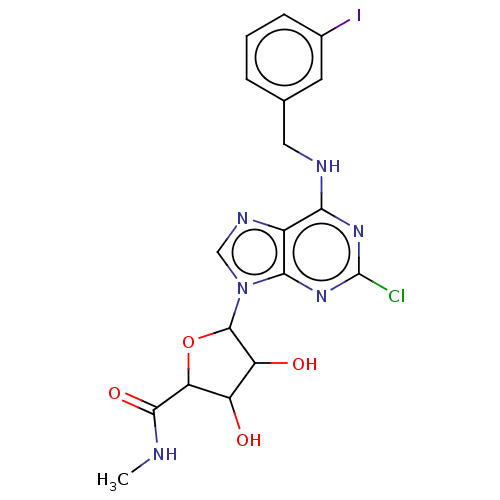 ((2S,3S,4R,5R)-5-[2-chloro-6-[(3-iodophenyl)methyla...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The membranes prepared from CHO cells transfected with human adenosine A3 receptors were used in binding assays. Nonspecific binding was determined i... | J Med Chem 51: 1764-70 (2008) Article DOI: 10.1021/jm701159t BindingDB Entry DOI: 10.7270/Q2MP51JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328980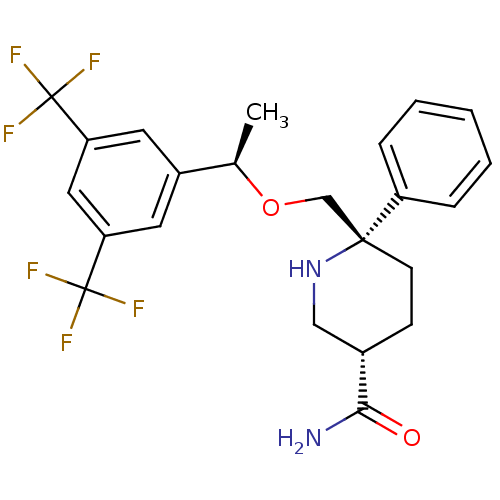 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 50: 5676-84 (2007) Article DOI: 10.1021/jm0708376 BindingDB Entry DOI: 10.7270/Q2SJ1MFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cell membrane after 90 mins | Eur J Med Chem 69: 331-7 (2013) Article DOI: 10.1016/j.ejmech.2013.09.001 BindingDB Entry DOI: 10.7270/Q28918TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane | J Med Chem 55: 1490-9 (2012) Article DOI: 10.1021/jm201177b BindingDB Entry DOI: 10.7270/Q2CR5VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50515869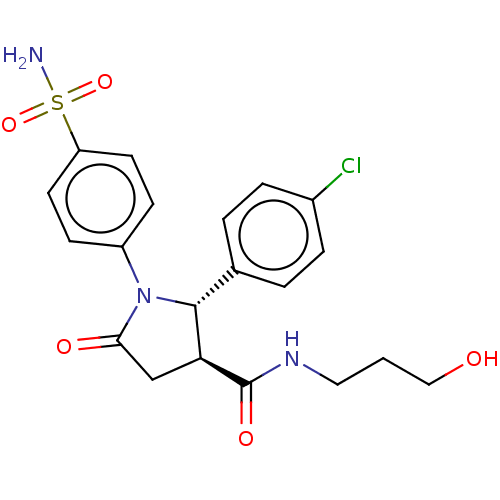 (CHEMBL4577408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111642 BindingDB Entry DOI: 10.7270/Q2C250SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328973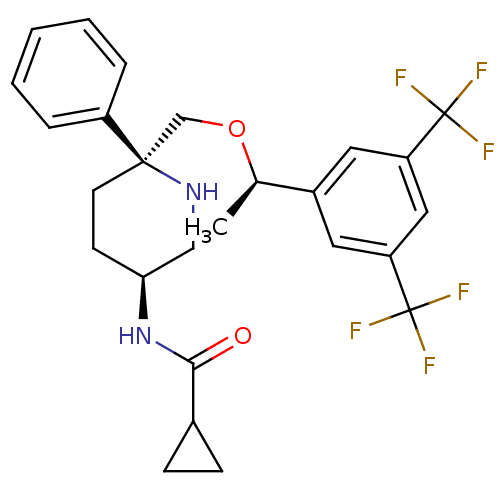 (CHEMBL1270175 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523351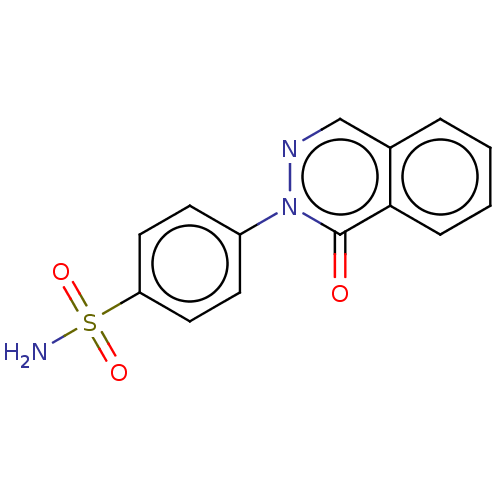 (CHEMBL4592596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253483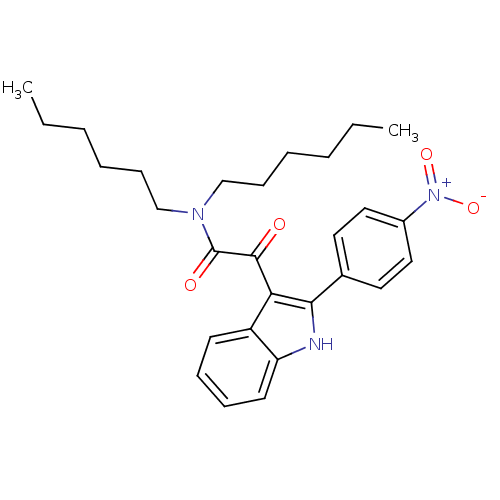 (CHEMBL492686 | N,N-dihexyl-2-(2-(4-nitrophenyl)-1H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50011484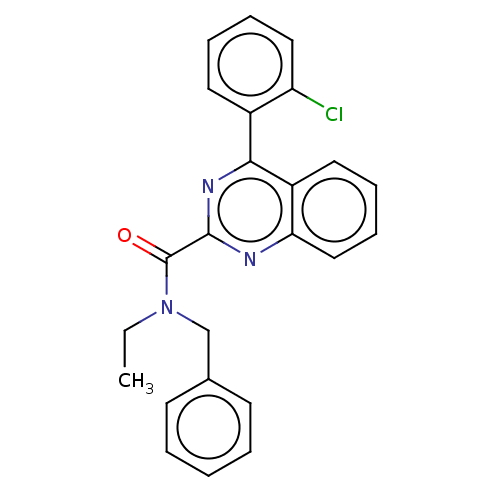 (CHEMBL3261894) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in rat kidney mitochondrial membranes | J Med Chem 57: 2413-28 (2014) Article DOI: 10.1021/jm401721h BindingDB Entry DOI: 10.7270/Q2416ZKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50523342 (CHEMBL4527427) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of membrane bound human CA 9 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50531512 (CHEMBL4552118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 assessed as reduction in CO2 hydration after 15 mins by phenol red staining-based stopped flow assay | Eur J Med Chem 164: 92-105 (2019) Article DOI: 10.1016/j.ejmech.2018.12.049 BindingDB Entry DOI: 10.7270/Q2R78JPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002875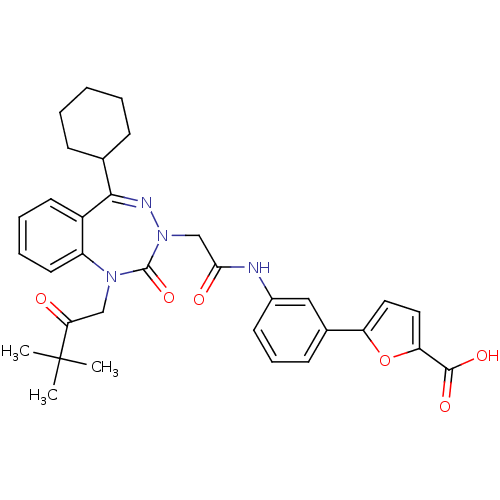 (CHEMBL388144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328974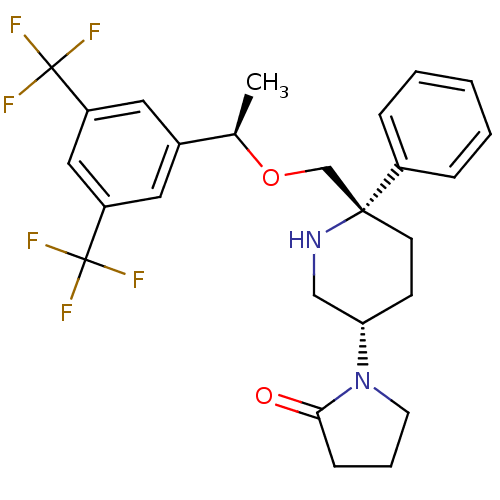 (1-((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50523349 (CHEMBL4466844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human cytosolic CA 2 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration assay | Eur J Med Chem 168: 301-314 (2019) Article DOI: 10.1016/j.ejmech.2019.02.044 BindingDB Entry DOI: 10.7270/Q21C218G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328982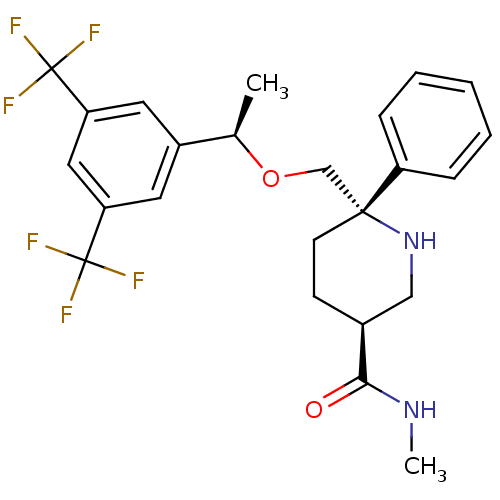 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253344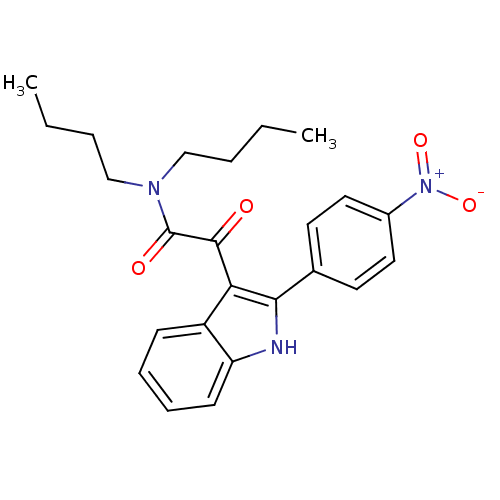 (CHEMBL493301 | N,N-dibutyl-2-(2-(4-nitrophenyl)-1H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411340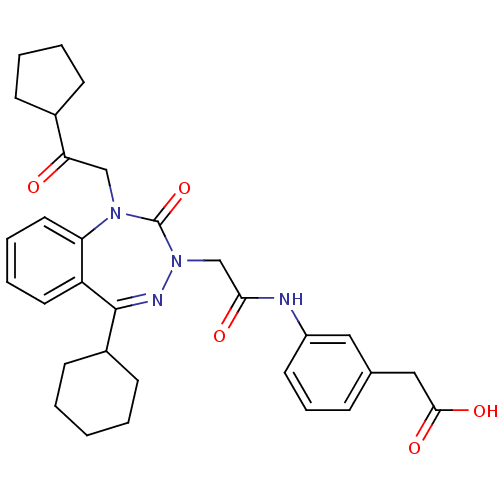 (CHEMBL387948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9858 total ) | Next | Last >> |