

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222089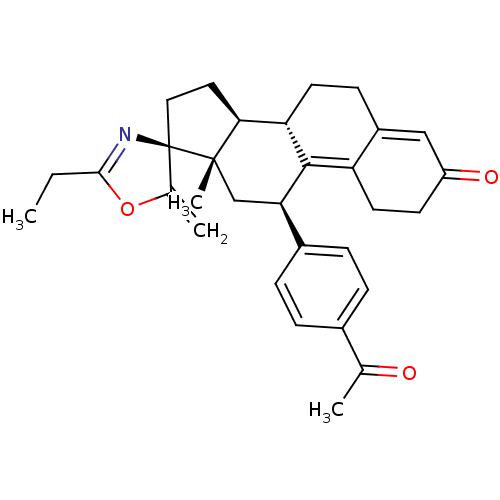 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222086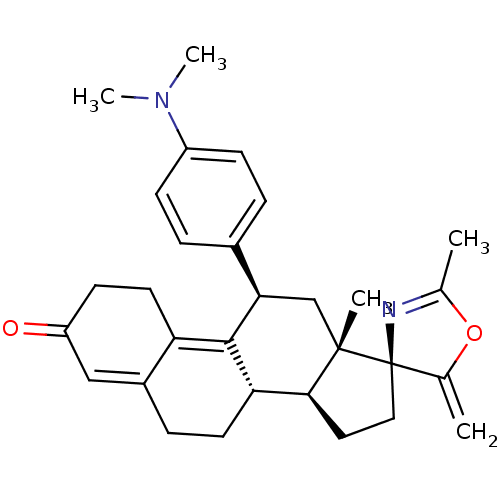 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222088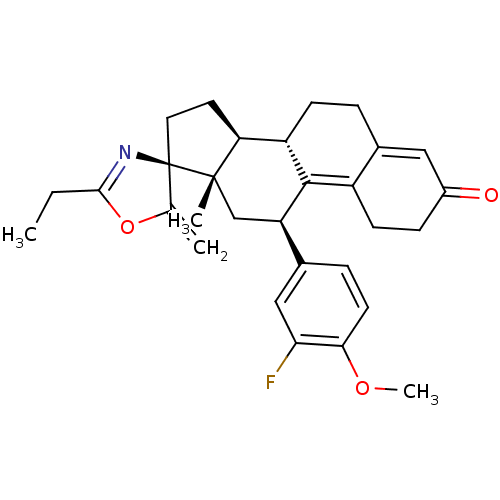 ((3S,10'S,11'S,15'S,17'R)-5-ethyl-17'-(3-fluoro-4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50201772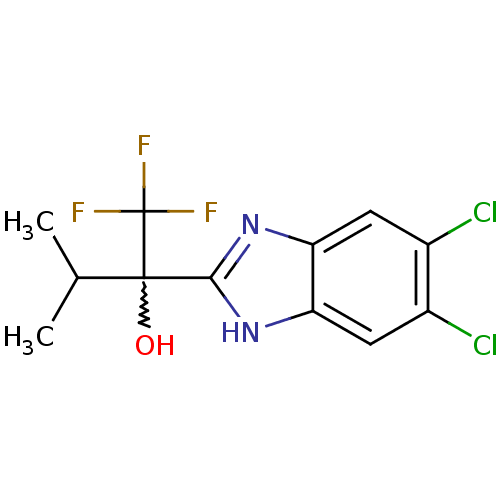 (2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,1-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at androgen receptor expressed in COS cells by whole cell binding assay | Bioorg Med Chem Lett 17: 1784-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.045 BindingDB Entry DOI: 10.7270/Q2KW5FQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222087 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50201773 (2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,1-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at androgen receptor expressed in COS cells by whole cell binding assay | Bioorg Med Chem Lett 17: 1784-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.045 BindingDB Entry DOI: 10.7270/Q2KW5FQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50201771 (2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-3-(ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at androgen receptor expressed in COS cells by whole cell binding assay | Bioorg Med Chem Lett 17: 1784-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.045 BindingDB Entry DOI: 10.7270/Q2KW5FQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222085 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185197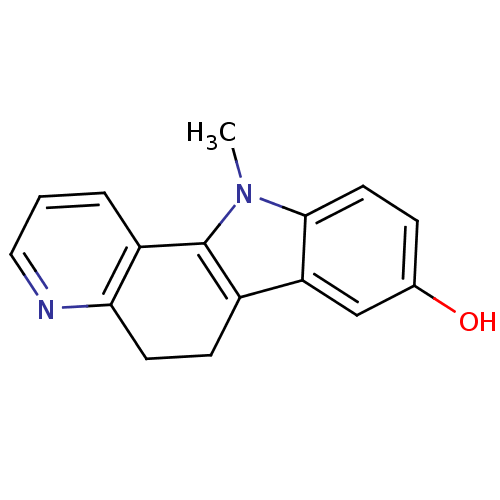 (11-methyl-6,11-dihydro-5H-pyrido[3,2-a]carbazol-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185206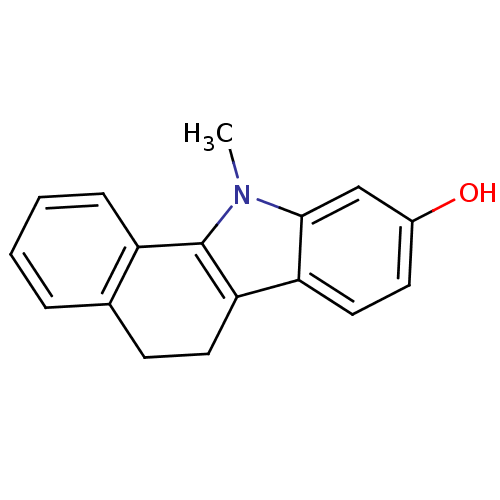 (11-methyl-6,11-dihydro-5H-benzo[a]carbazol-9-ol | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185194 (11-methyl-6,11-dihydro-5-thia-4,11-diaza-benzo[a]f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222090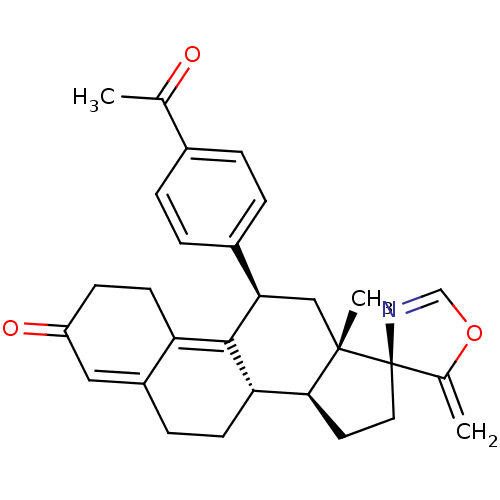 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-15'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222086 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194351 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185210 (11-methyl-6,11-dihydrothiochromeno[4,3-b]indol-8-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185207 (2-fluoro-11-methyl-6,11-dihydrothiochromeno[4,3-b]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185191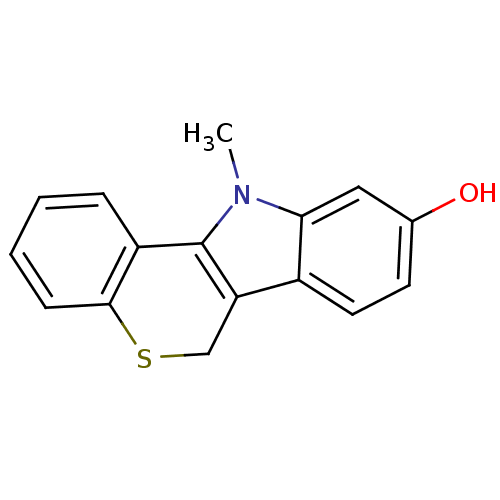 (11-methyl-6,11-dihydrothiochromeno[4,3-b]indol-9-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194342 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194331 (2-hydroxy-2-(5-(2,2,2-trifluoroacetamido)-6-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194352 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194337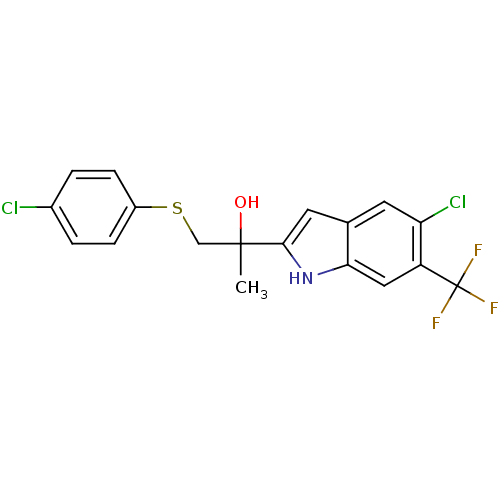 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194350 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222089 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194330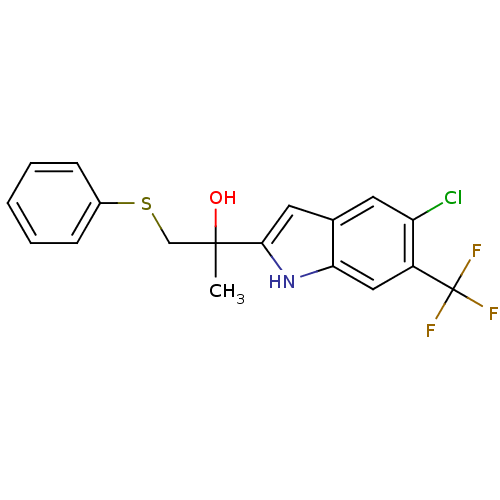 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185202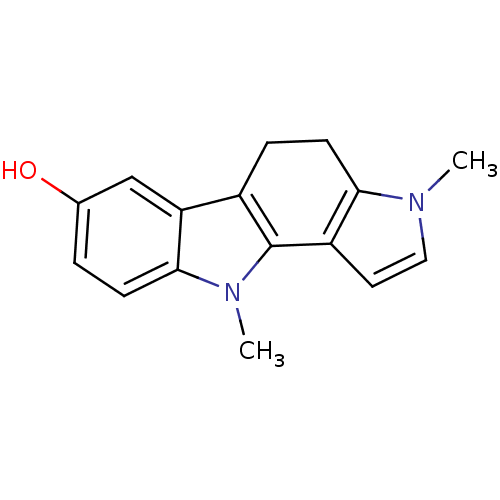 (3,10-dimethyl-3,4,5,10-tetrahydropyrrolo[3,2-a]car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194353 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194326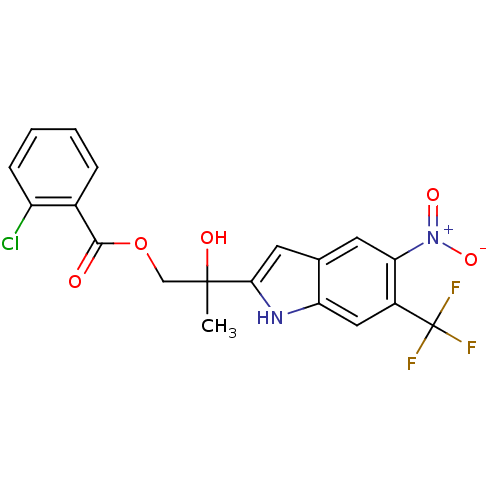 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194340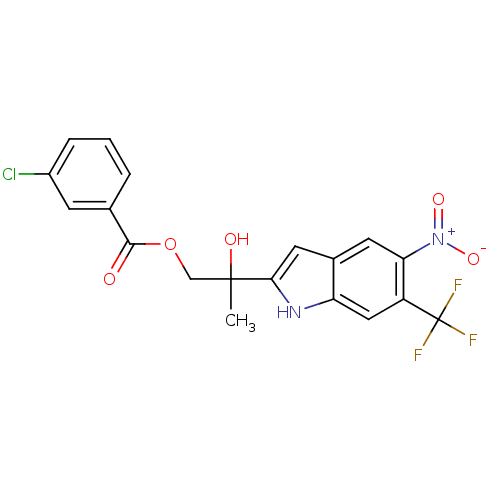 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194345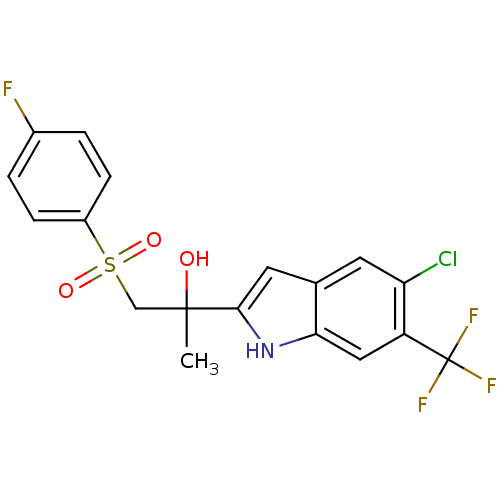 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194327 (2-(5-cyano-6-(trifluoromethyl)-1H-indol-2-yl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222085 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185190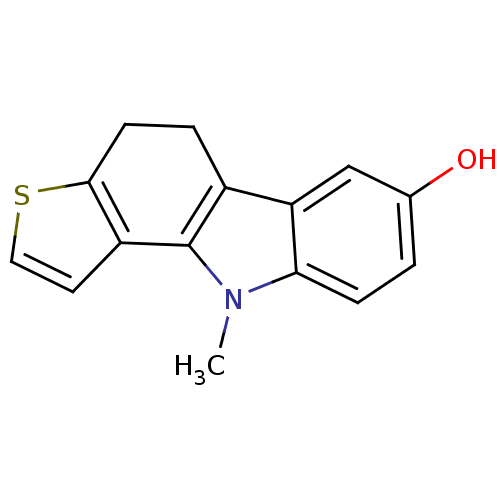 (10-methyl-5,10-dihydro-4H-thieno[3,2-a]carbazol-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185209 (3-fluoro-11-methyl-6,11-dihydrothiochromeno[4,3-b]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194354 (2-(5-chloro-1H-indol-2-yl)-1-(4-chlorophenylthio)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50222087 ((3S,10'S,11'S,15'S,17'R)-17'-(3-fluoro-4-methoxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in A549 cells assessed as inhibition of corticoid-induced gene transcription by luciferase r... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194344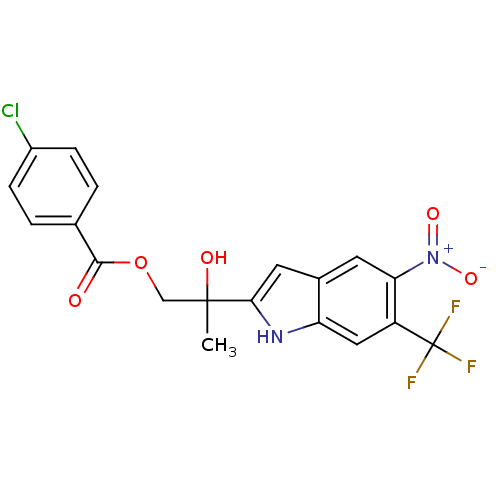 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194346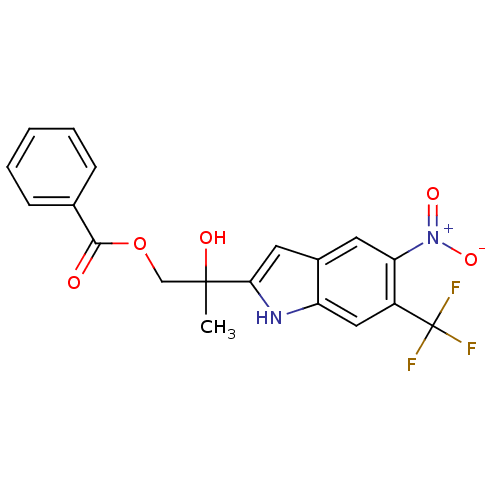 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18525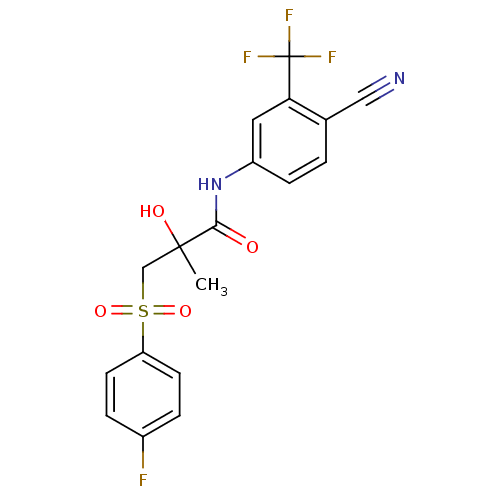 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185201 (11-methyl-6,11-dihydrothiochromeno[4,3-b]indol-8-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185213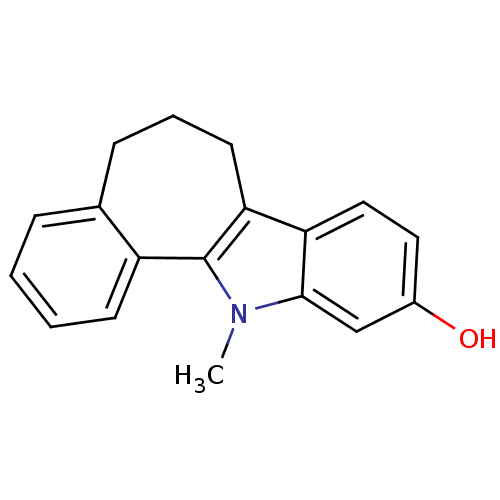 (12-methyl-5,6,7,12-tetrahydro-benzo[6,7]cyclohepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194328 (1-(4-aminophenylthio)-2-(5-chloro-6-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50185199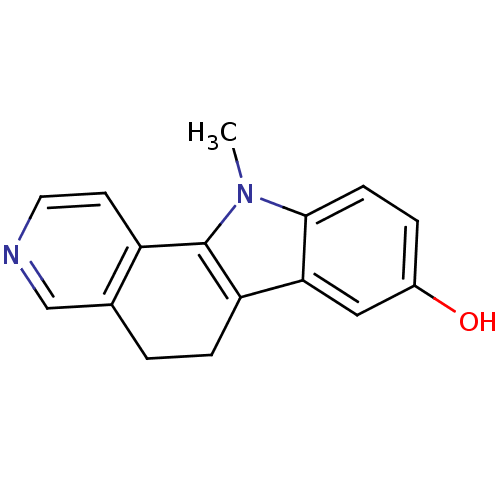 (11-methyl-6,11-dihydro-5H-pyrido[4,3-a]carbazol-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of rat AR-mediated reporter gene expression in COS7 cells | Bioorg Med Chem Lett 16: 3233-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.047 BindingDB Entry DOI: 10.7270/Q2Q52QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194339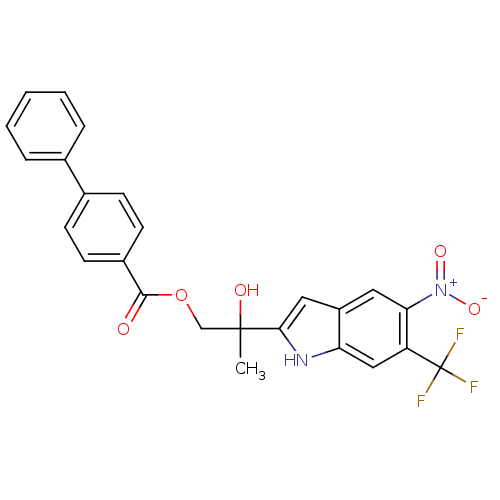 (CHEMBL376323 | biphenyl-4-carboxylic acid 2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |