

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50366775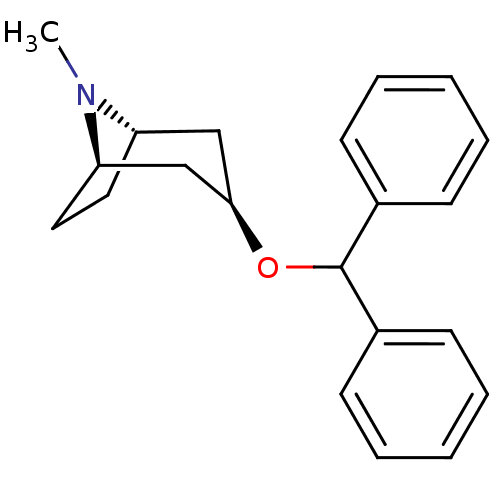 (BENZTROPINE | Benzatropine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453903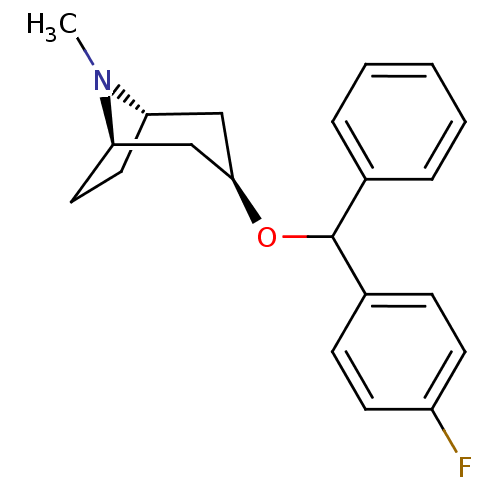 (CHEMBL3084883) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453899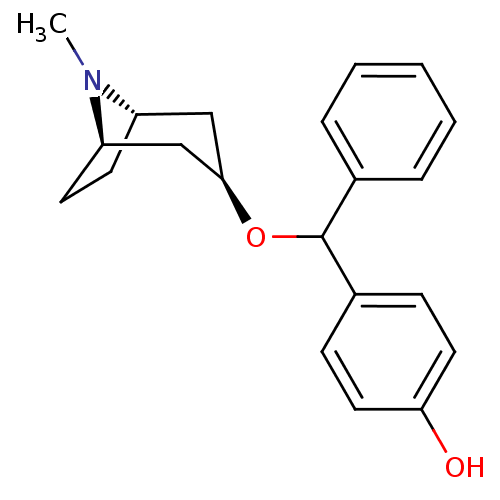 (CHEMBL3084872) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50366775 (BENZTROPINE | Benzatropine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453908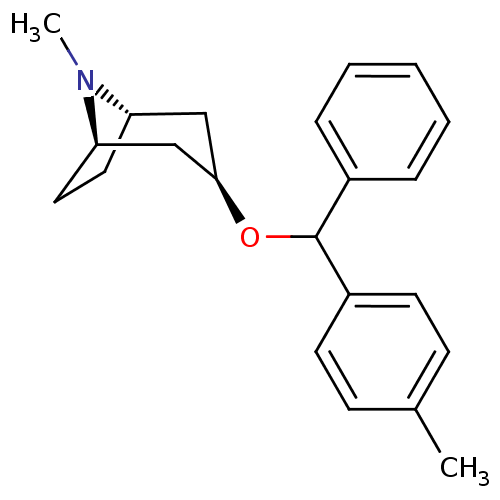 (CHEMBL3084881) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM86702 (3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453905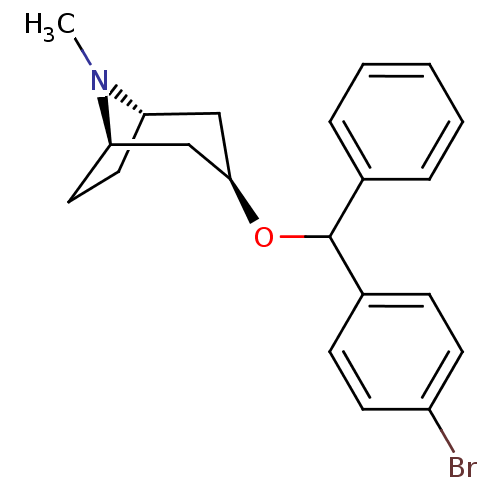 (CHEMBL3084882) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM86702 (3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453909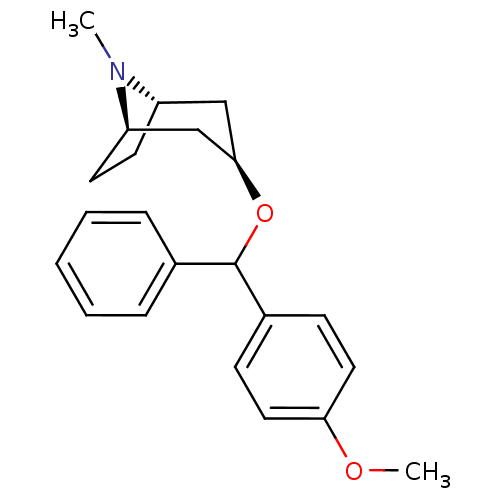 (CHEMBL3084880) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453906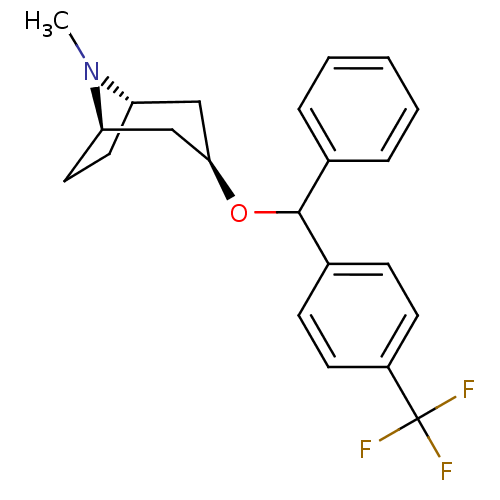 (CHEMBL3084873) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM86701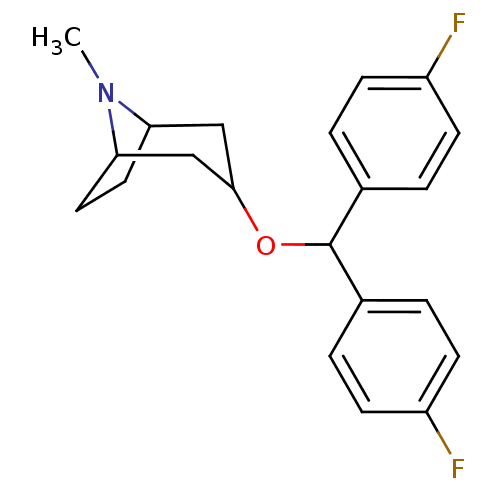 (3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453911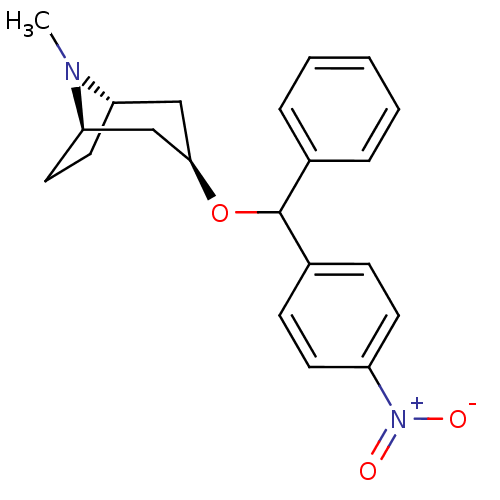 (CHEMBL3084899) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453907 (CHEMBL3084900) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM86701 (3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]WIN-35428 binding in rat caudate-putamen | J Med Chem 37: 2258-61 (1994) BindingDB Entry DOI: 10.7270/Q2FJ2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165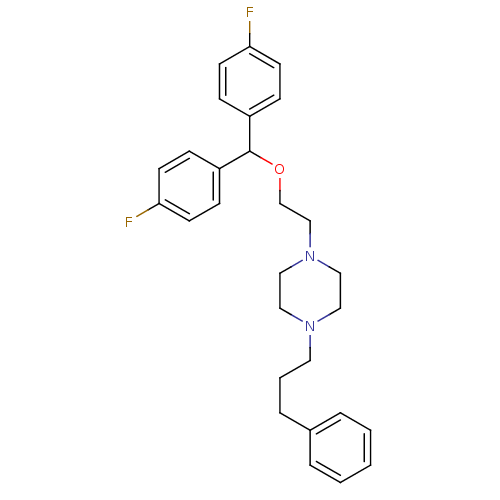 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM86701 (3-(bis(4-fluorophenyl)methoxy)-8-methyl-8-aza-bicy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]WIN-35428 binding in rat caudate-putamen | J Med Chem 37: 2258-61 (1994) BindingDB Entry DOI: 10.7270/Q2FJ2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453904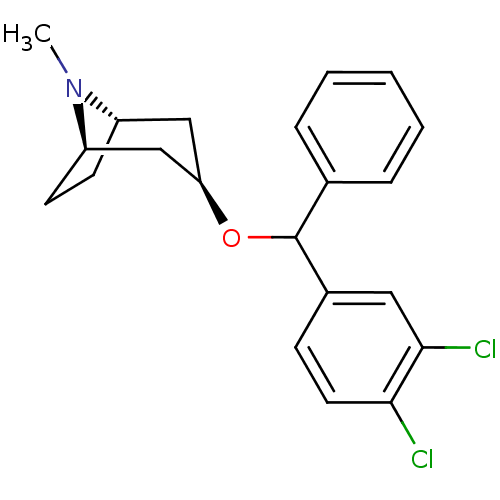 (CHEMBL3084892) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453896 (CHEMBL3084898) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282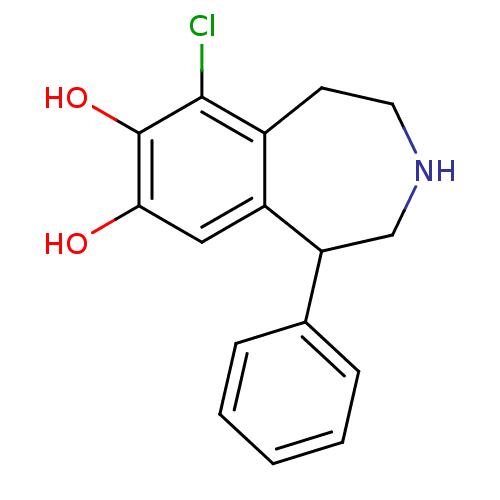 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453903 (CHEMBL3084883) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453899 (CHEMBL3084872) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453909 (CHEMBL3084880) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453910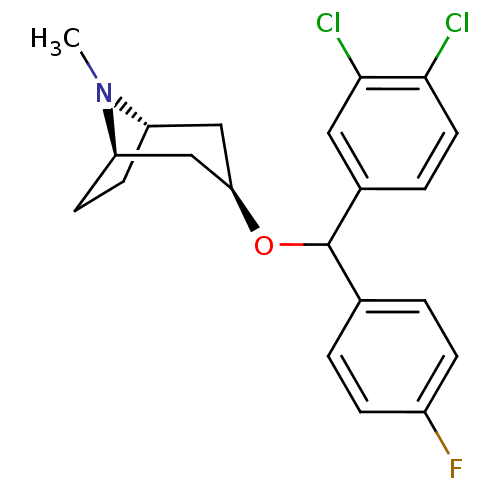 (CHEMBL3084896) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM223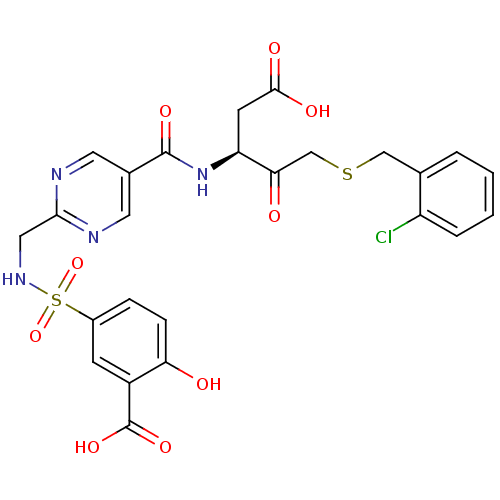 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM226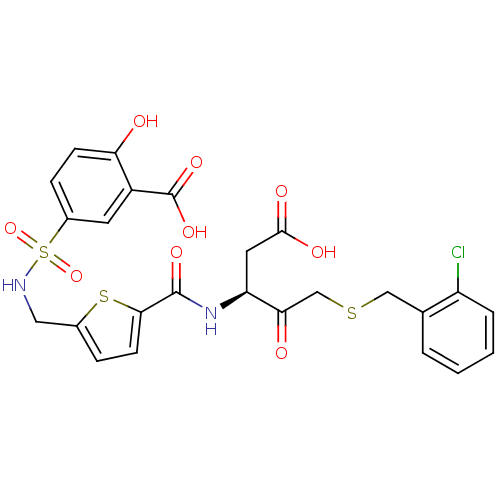 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50039177 (3-[Bis-(4-chloro-phenyl)-methoxy]-8-methyl-8-aza-b...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]WIN-35428 binding in rat caudate-putamen | J Med Chem 37: 2258-61 (1994) BindingDB Entry DOI: 10.7270/Q2FJ2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453902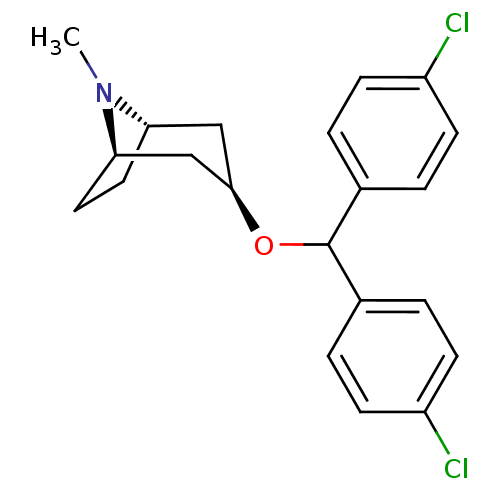 (CHEMBL3084884) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM263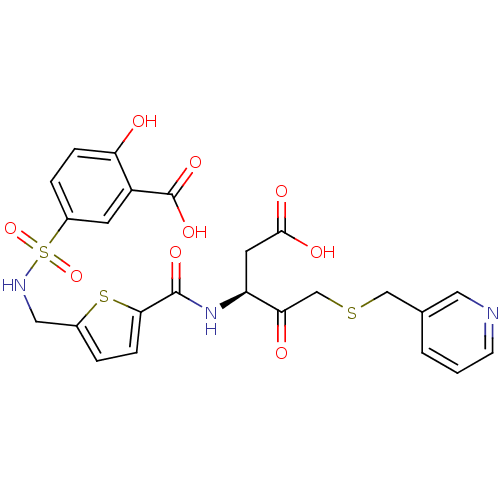 ((S)-5-({5-[1-Carboxymethyl-2-oxo-3-(pyridin-3-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM280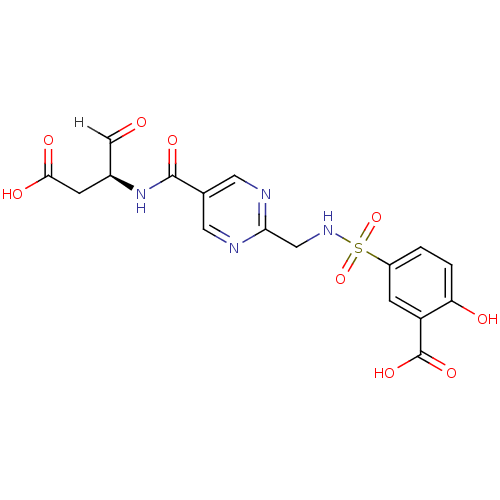 ((S)-5-{[5-(1-Carboxymethyl-2-oxo-ethylcarbamoyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453904 (CHEMBL3084892) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM86702 (3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM224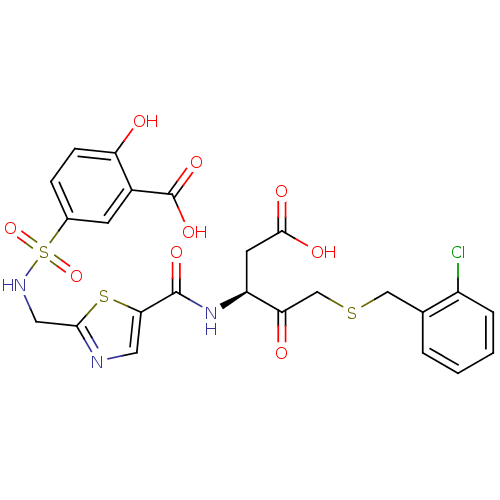 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM222 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM264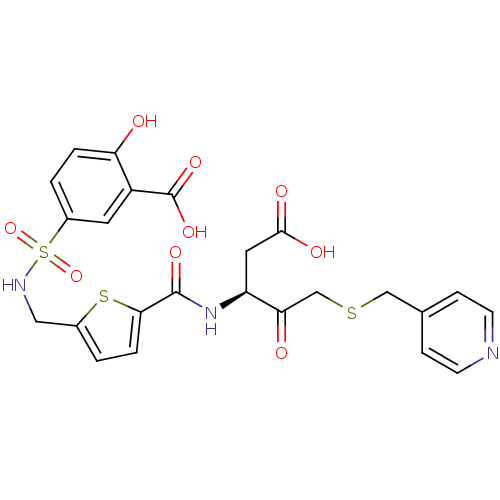 ((S)-5-({5-[1-Carboxymethyl-2-oxo-3-(pyridin-4-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453376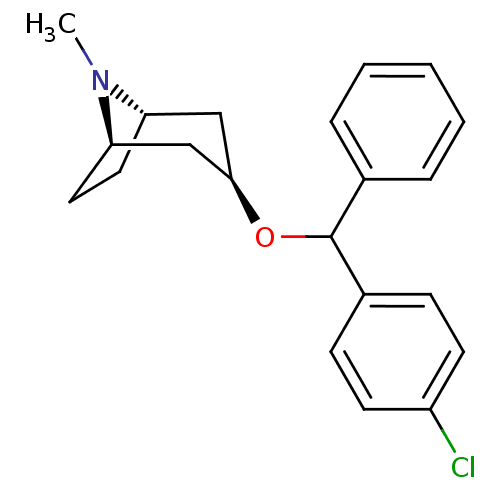 (CHEMBL3085569) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]WIN-35428 binding in rat caudate-putamen | J Med Chem 37: 2258-61 (1994) BindingDB Entry DOI: 10.7270/Q2FJ2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM86702 (3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453903 (CHEMBL3084883) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717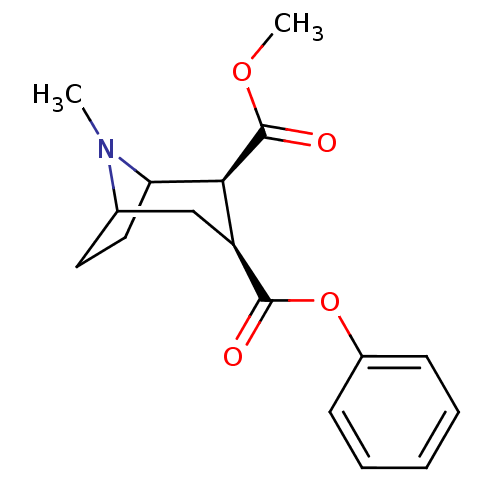 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453910 (CHEMBL3084896) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453902 (CHEMBL3084884) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM86702 (3-[(4-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50453905 (CHEMBL3084882) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat dopamine transporter using [3H]WIN-35428 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453908 (CHEMBL3084881) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453905 (CHEMBL3084882) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM227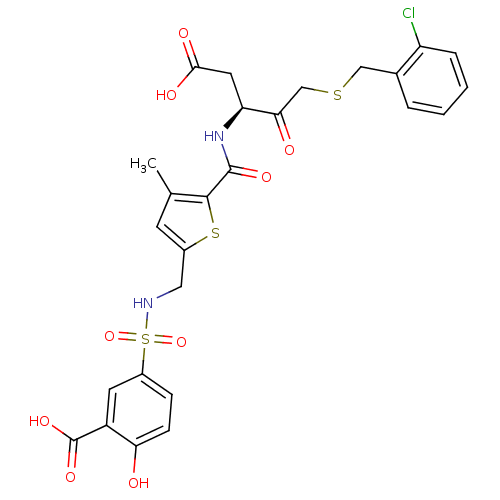 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453901 (CHEMBL3084867) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50453911 (CHEMBL3084899) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]-AF DX 384 displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453898 (CHEMBL3084888) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 895 total ) | Next | Last >> |