 Found 1175 hits with Last Name = 'allen' and Initial = 'jg'
Found 1175 hits with Last Name = 'allen' and Initial = 'jg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50101858
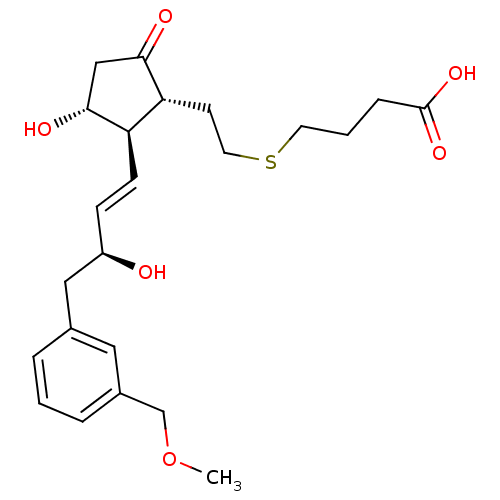
(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(O)=O)c1 Show InChI InChI=1S/C23H32O6S/c1-29-15-17-5-2-4-16(12-17)13-18(24)7-8-19-20(22(26)14-21(19)25)9-11-30-10-3-6-23(27)28/h2,4-5,7-8,12,18-21,24-25H,3,6,9-11,13-15H2,1H3,(H,27,28)/b8-7+/t18-,19-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP4 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318881

(CHEMBL1086008 | N-(1-((2S,3S)-2-ethyl-4-oxotetrahy...)Show SMILES CC[C@@H]1OCC(=O)[C@H]1NC(=O)C1(CCCCC1)NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C28H37N5O4S/c1-3-23-24(22(34)17-37-23)30-26(36)28(11-5-4-6-12-28)31-25(35)20-9-7-19(8-10-20)21-18-38-27(29-21)33-15-13-32(2)14-16-33/h7-10,18,23-24H,3-6,11-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036
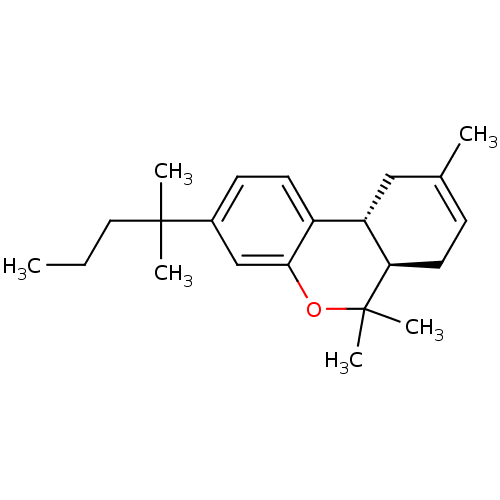
((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318882
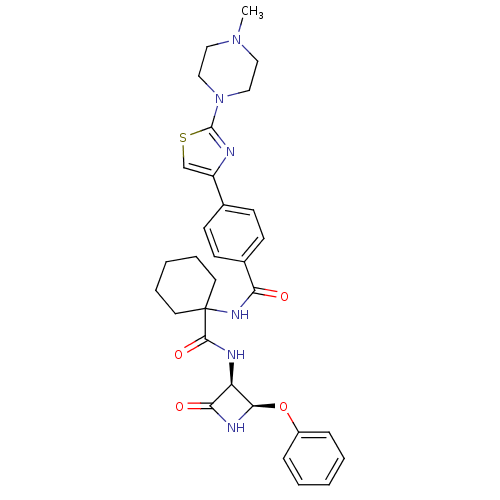
(4-(2-(4-methylpiperazin-1-yl)thiazol-4-yl)-N-(1-((...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)N[C@H]1[C@H](NC1=O)Oc1ccccc1 |r| Show InChI InChI=1S/C31H36N6O4S/c1-36-16-18-37(19-17-36)30-32-24(20-42-30)21-10-12-22(13-11-21)26(38)35-31(14-6-3-7-15-31)29(40)33-25-27(39)34-28(25)41-23-8-4-2-5-9-23/h2,4-5,8-13,20,25,28H,3,6-7,14-19H2,1H3,(H,33,40)(H,34,39)(H,35,38)/t25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279
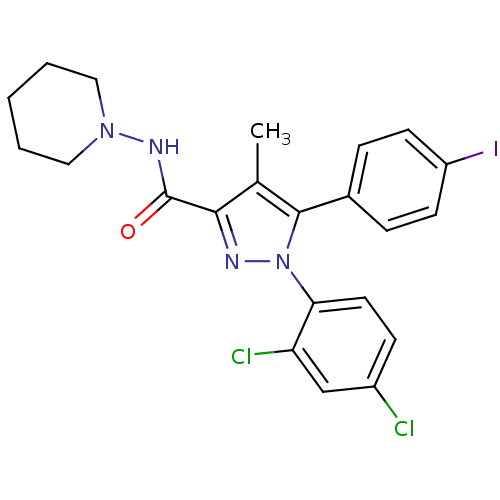
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318879
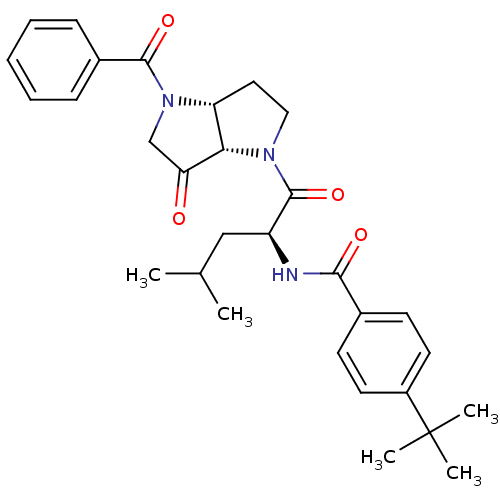
(CHEMBL607169 | N-((S)-1-((3aR,6aS)-4-benzoyl-6-oxo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1CC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 Show InChI InChI=1S/C30H37N3O4/c1-19(2)17-23(31-27(35)20-11-13-22(14-12-20)30(3,4)5)29(37)32-16-15-24-26(32)25(34)18-33(24)28(36)21-9-7-6-8-10-21/h6-14,19,23-24,26H,15-18H2,1-5H3,(H,31,35)/t23-,24+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K at enzyme-substrate complex state |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50265621
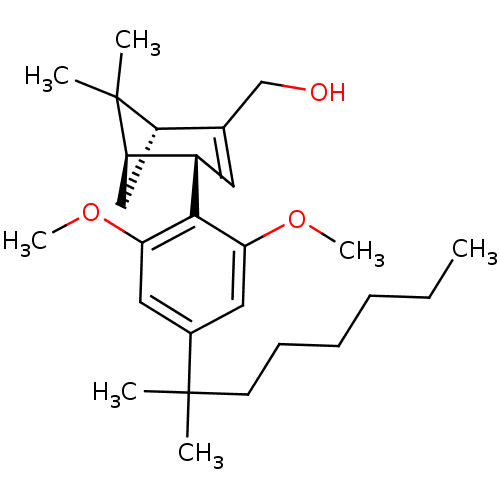
(((1R,4S,5R)-4-(2,6-dimethoxy-4-(2-methyloctan-2-yl...)Show SMILES CCCCCCC(C)(C)c1cc(OC)c([C@H]2C=C(CO)[C@@H]3C[C@H]2C3(C)C)c(OC)c1 |r,t:16| Show InChI InChI=1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160434
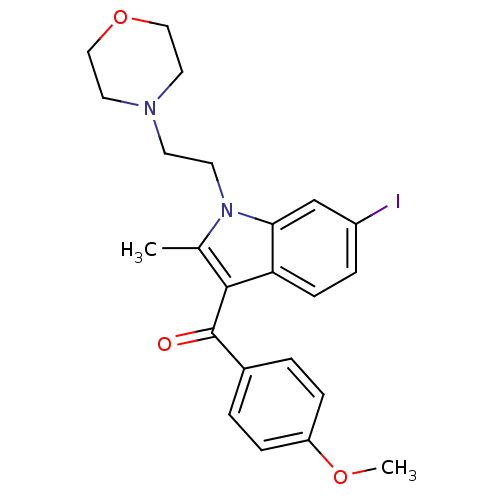
((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50318871

(4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(=O)OC)c1 |r| Show InChI InChI=1S/C24H34O6S/c1-29-16-18-6-3-5-17(13-18)14-19(25)8-9-20-21(23(27)15-22(20)26)10-12-31-11-4-7-24(28)30-2/h3,5-6,8-9,13,19-22,25-26H,4,7,10-12,14-16H2,1-2H3/b9-8+/t19-,20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP3 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(O)=O)c1 Show InChI InChI=1S/C23H32O6S/c1-29-15-17-5-2-4-16(12-17)13-18(24)7-8-19-20(22(26)14-21(19)25)9-11-30-10-3-6-23(27)28/h2,4-5,7-8,12,18-21,24-25H,3,6,9-11,13-15H2,1H3,(H,27,28)/b8-7+/t18-,19-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse EP2 receptor by competitive binding assay |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50180036

((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 677 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21279

(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160434

((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in CHO cells |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50191290

(3-((5-(2-chloro-4,5-dimethoxybenzylidene)-3-methyl...)Show SMILES COc1cc(Cl)c(\C=C2/S\C(=N\c3cccc(c3)C(O)=O)N(C)C2=O)cc1OC Show InChI InChI=1S/C20H17ClN2O5S/c1-23-18(24)17(9-12-8-15(27-2)16(28-3)10-14(12)21)29-20(23)22-13-6-4-5-11(7-13)19(25)26/h4-10H,1-3H3,(H,25,26)/b17-9-,22-20+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]echistatin from integrin alphaVbeta3 in human NCI-H1975 cells after 3 hrs by gamma counting |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50134778

((S)-3-(6-Methoxy-pyridin-3-yl)-3-{2-oxo-3-[3-(5,6,...)Show SMILES COc1ccc(cn1)[C@H](CC(O)=O)N1CCN(CCCc2ccc3CCCNc3n2)C1=O Show InChI InChI=1S/C23H29N5O4/c1-32-20-9-7-17(15-25-20)19(14-21(29)30)28-13-12-27(23(28)31)11-3-5-18-8-6-16-4-2-10-24-22(16)26-18/h6-9,15,19H,2-5,10-14H2,1H3,(H,24,26)(H,29,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50318878
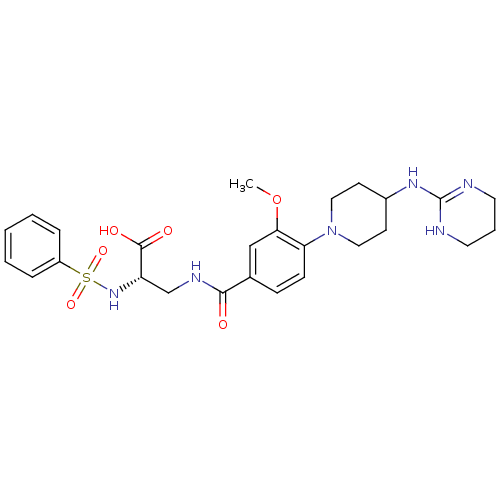
((S)-3-(3-methoxy-4-(4-(1,4,5,6-tetrahydropyrimidin...)Show SMILES COc1cc(ccc1N1CCC(CC1)NC1=NCCCN1)C(=O)NC[C@H](NS(=O)(=O)c1ccccc1)C(O)=O |r,t:17| Show InChI InChI=1S/C26H34N6O6S/c1-38-23-16-18(8-9-22(23)32-14-10-19(11-15-32)30-26-27-12-5-13-28-26)24(33)29-17-21(25(34)35)31-39(36,37)20-6-3-2-4-7-20/h2-4,6-9,16,19,21,31H,5,10-15,17H2,1H3,(H,29,33)(H,34,35)(H2,27,28,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753
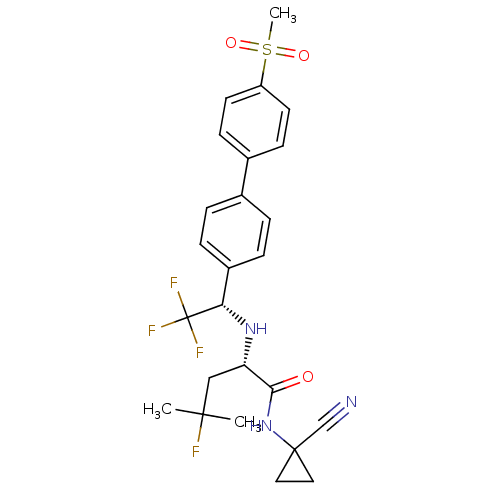
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50162757

((3S,7S)-7-Hydroxy-3-(6-methoxy-pyridin-3-yl)-9-(5,...)Show SMILES COc1ccc(cn1)[C@@H](CCC[C@H](O)CCc1ccc2CCCNc2n1)CC(O)=O Show InChI InChI=1S/C23H31N3O4/c1-30-21-12-8-18(15-25-21)17(14-22(28)29)4-2-6-20(27)11-10-19-9-7-16-5-3-13-24-23(16)26-19/h7-9,12,15,17,20,27H,2-6,10-11,13-14H2,1H3,(H,24,26)(H,28,29)/t17-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177628
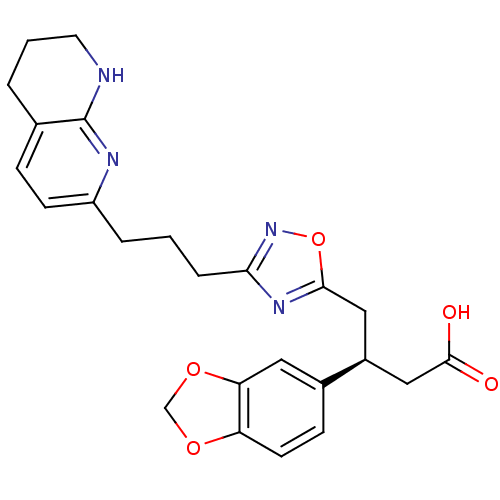
((S)-3-(benzo[d][1,3]dioxol-5-yl)-4-(3-(3-(5,6,7,8-...)Show SMILES OC(=O)C[C@H](Cc1nc(CCCc2ccc3CCCNc3n2)no1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H26N4O5/c29-23(30)13-17(16-7-9-19-20(11-16)32-14-31-19)12-22-27-21(28-33-22)5-1-4-18-8-6-15-3-2-10-25-24(15)26-18/h6-9,11,17H,1-5,10,12-14H2,(H,25,26)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50201116
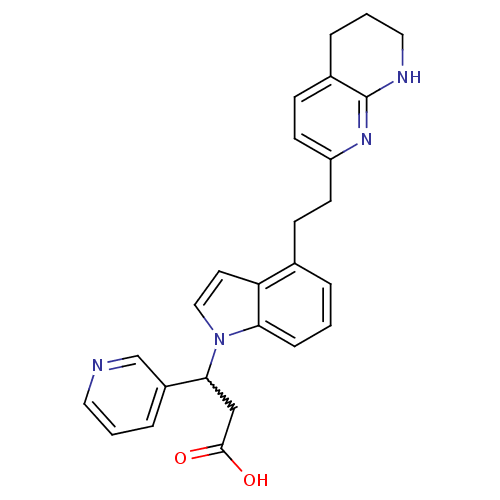
(3-(3-pyridyl)-3-[4-[2-(5,6,7,8-tetrahydro[1,8]naph...)Show SMILES OC(=O)CC(c1cccnc1)n1ccc2c(CCc3ccc4CCCNc4n3)cccc12 |w:4.3| Show InChI InChI=1S/C26H26N4O2/c31-25(32)16-24(20-6-2-13-27-17-20)30-15-12-22-18(4-1-7-23(22)30)8-10-21-11-9-19-5-3-14-28-26(19)29-21/h1-2,4,6-7,9,11-13,15,17,24H,3,5,8,10,14,16H2,(H,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50235980

(2-((2S,5R,8S,11S)-5-benzyl-11-(3-guanidinopropyl)-...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7](-[#6])-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50253098
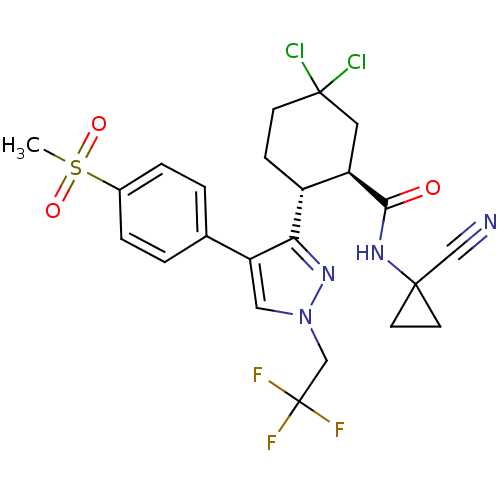
((1R,2R)-5,5-Dichloro-N-(1-cyanocyclopropyl)-2-[4-[...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cn(CC(F)(F)F)nc1[C@@H]1CCC(Cl)(Cl)C[C@H]1C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H23Cl2F3N4O3S/c1-36(34,35)15-4-2-14(3-5-15)18-11-32(13-23(26,27)28)31-19(18)16-6-7-22(24,25)10-17(16)20(33)30-21(12-29)8-9-21/h2-5,11,16-17H,6-10,13H2,1H3,(H,30,33)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443648
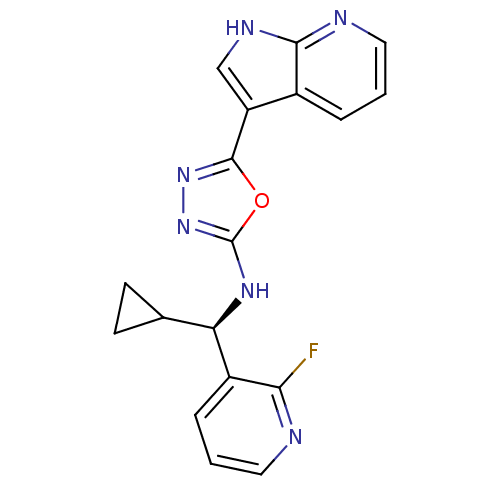
(CHEMBL3093076)Show SMILES Fc1ncccc1[C@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)C1CC1 |r| Show InChI InChI=1S/C18H15FN6O/c19-15-12(4-2-7-20-15)14(10-5-6-10)23-18-25-24-17(26-18)13-9-22-16-11(13)3-1-8-21-16/h1-4,7-10,14H,5-6H2,(H,21,22)(H,23,25)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443649
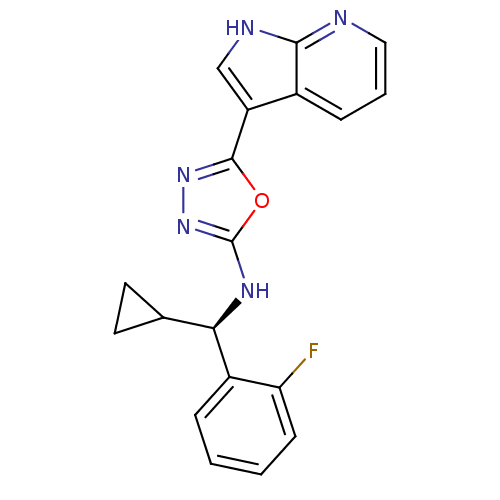
(CHEMBL3093075)Show SMILES Fc1ccccc1[C@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)C1CC1 |r| Show InChI InChI=1S/C19H16FN5O/c20-15-6-2-1-4-13(15)16(11-7-8-11)23-19-25-24-18(26-19)14-10-22-17-12(14)5-3-9-21-17/h1-6,9-11,16H,7-8H2,(H,21,22)(H,23,25)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443650
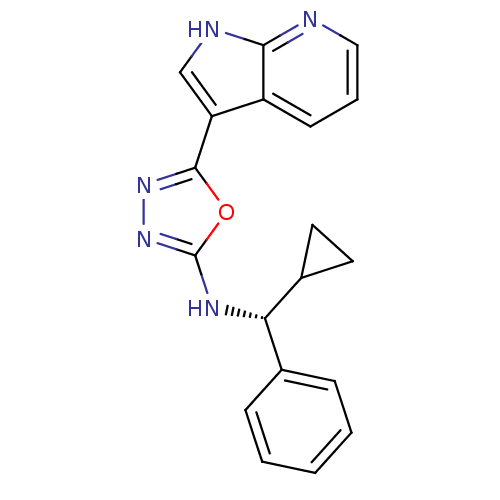
(CHEMBL3093074)Show SMILES C1CC1[C@@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)c1ccccc1 |r| Show InChI InChI=1S/C19H17N5O/c1-2-5-12(6-3-1)16(13-8-9-13)22-19-24-23-18(25-19)15-11-21-17-14(15)7-4-10-20-17/h1-7,10-11,13,16H,8-9H2,(H,20,21)(H,22,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443644
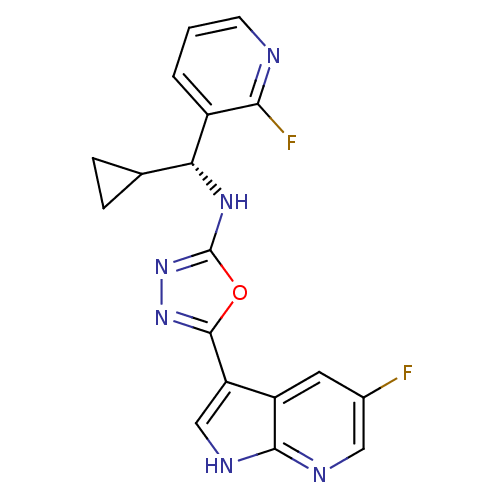
(CHEMBL3093080)Show SMILES Fc1cnc2[nH]cc(-c3nnc(N[C@H](C4CC4)c4cccnc4F)o3)c2c1 |r| Show InChI InChI=1S/C18H14F2N6O/c19-10-6-12-13(8-23-16(12)22-7-10)17-25-26-18(27-17)24-14(9-3-4-9)11-2-1-5-21-15(11)20/h1-2,5-9,14H,3-4H2,(H,22,23)(H,24,26)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698
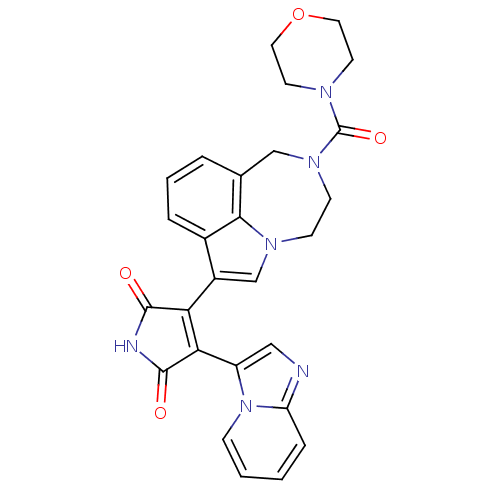
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19855
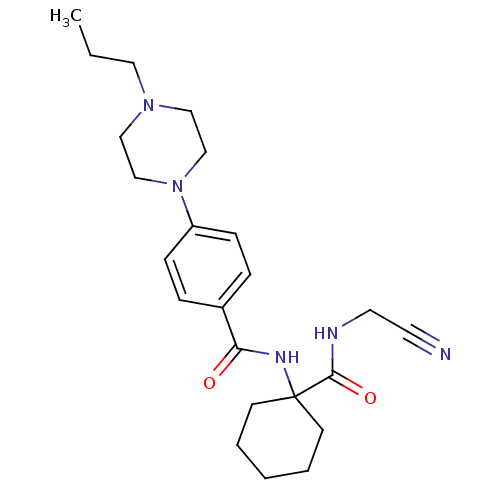
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443646
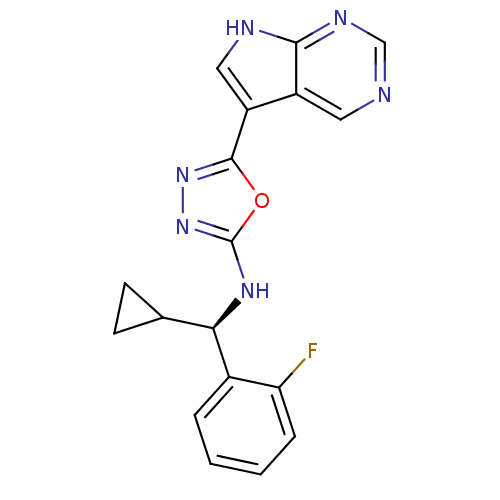
(CHEMBL3093078)Show SMILES Fc1ccccc1[C@H](Nc1nnc(o1)-c1c[nH]c2ncncc12)C1CC1 |r| Show InChI InChI=1S/C18H15FN6O/c19-14-4-2-1-3-11(14)15(10-5-6-10)23-18-25-24-17(26-18)13-8-21-16-12(13)7-20-9-22-16/h1-4,7-10,15H,5-6H2,(H,23,25)(H,20,21,22)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060
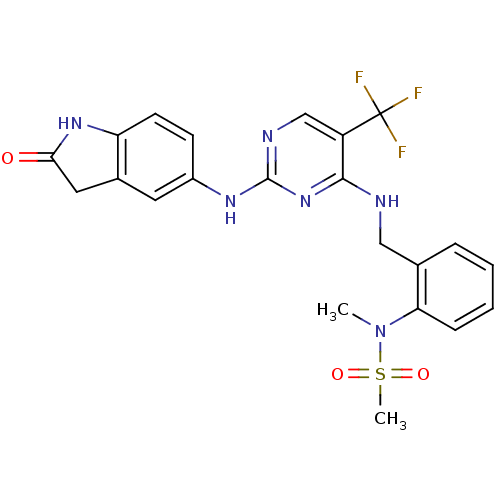
(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443647
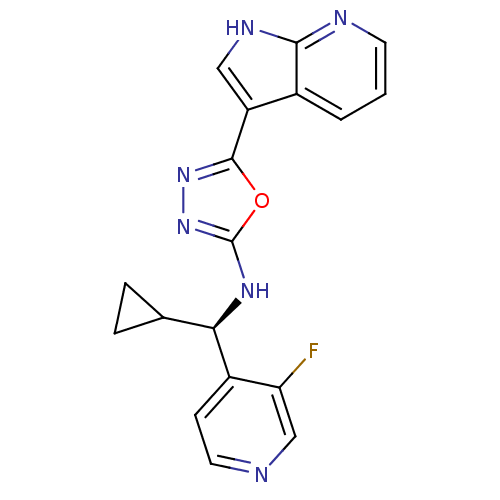
(CHEMBL3093077)Show SMILES Fc1cnccc1[C@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)C1CC1 |r| Show InChI InChI=1S/C18H15FN6O/c19-14-9-20-7-5-12(14)15(10-3-4-10)23-18-25-24-17(26-18)13-8-22-16-11(13)2-1-6-21-16/h1-2,5-10,15H,3-4H2,(H,21,22)(H,23,25)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443651
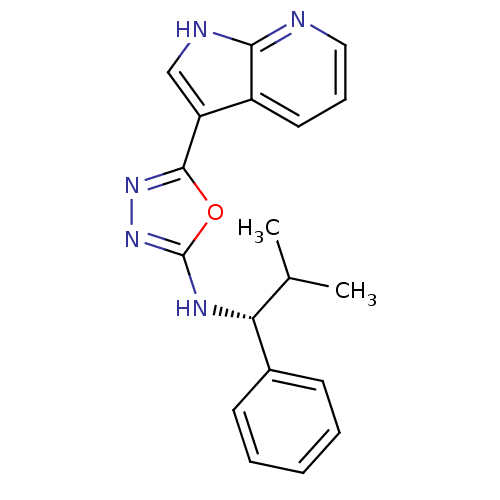
(CHEMBL3093073)Show SMILES CC(C)[C@@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)c1ccccc1 |r| Show InChI InChI=1S/C19H19N5O/c1-12(2)16(13-7-4-3-5-8-13)22-19-24-23-18(25-19)15-11-21-17-14(15)9-6-10-20-17/h3-12,16H,1-2H3,(H,20,21)(H,22,24)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50312752
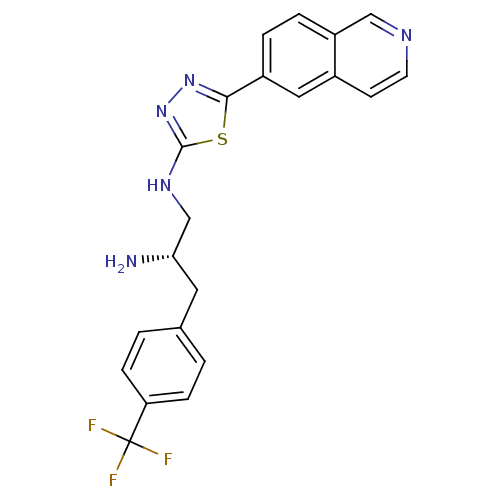
((S)-N1-(5-(isoquinolin-6-yl)-1,3,4-thiadiazol-2-yl...)Show SMILES N[C@H](CNc1nnc(s1)-c1ccc2cnccc2c1)Cc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C21H18F3N5S/c22-21(23,24)17-5-1-13(2-6-17)9-18(25)12-27-20-29-28-19(30-20)15-3-4-16-11-26-8-7-14(16)10-15/h1-8,10-11,18H,9,12,25H2,(H,27,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 21: 5191-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.056
BindingDB Entry DOI: 10.7270/Q21G0MNQ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50312752

((S)-N1-(5-(isoquinolin-6-yl)-1,3,4-thiadiazol-2-yl...)Show SMILES N[C@H](CNc1nnc(s1)-c1ccc2cnccc2c1)Cc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C21H18F3N5S/c22-21(23,24)17-5-1-13(2-6-17)9-18(25)12-27-20-29-28-19(30-20)15-3-4-16-11-26-8-7-14(16)10-15/h1-8,10-11,18H,9,12,25H2,(H,27,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 in human U87MG cells assessed as PRAS40 phosphorylation after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1559-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.067
BindingDB Entry DOI: 10.7270/Q20C4VWX |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50016163
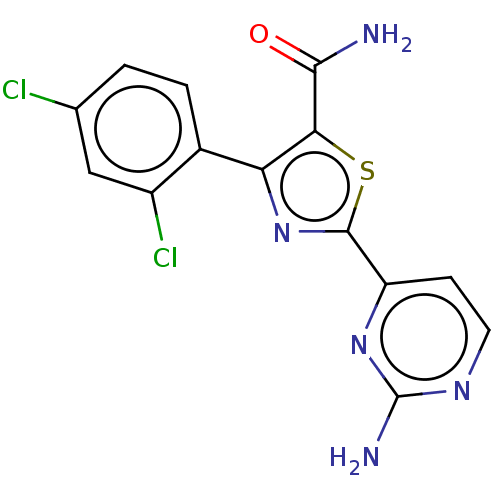
(CHEMBL3261514)Show InChI InChI=1S/C14H9Cl2N5OS/c15-6-1-2-7(8(16)5-6)10-11(12(17)22)23-13(21-10)9-3-4-19-14(18)20-9/h1-5H,(H2,17,22)(H2,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated phosphorylation of MCM2 by protein-A luminescent proximity homogenous assay |
Eur J Med Chem 80: 364-82 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.013
BindingDB Entry DOI: 10.7270/Q2NC62R8 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443645
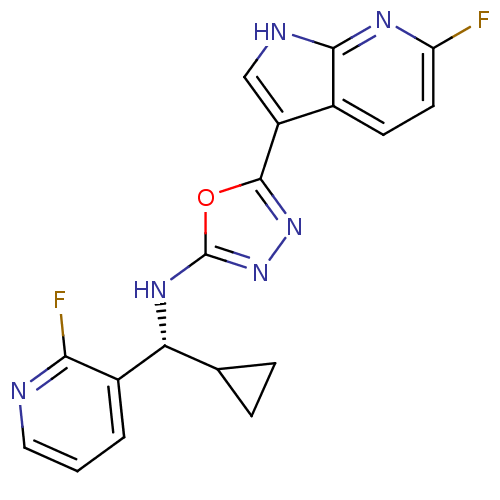
(CHEMBL3093079)Show SMILES Fc1ccc2c(c[nH]c2n1)-c1nnc(N[C@H](C2CC2)c2cccnc2F)o1 |r| Show InChI InChI=1S/C18H14F2N6O/c19-13-6-5-10-12(8-22-16(10)23-13)17-25-26-18(27-17)24-14(9-3-4-9)11-2-1-7-21-15(11)20/h1-2,5-9,14H,3-4H2,(H,22,23)(H,24,26)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of YES |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50443652
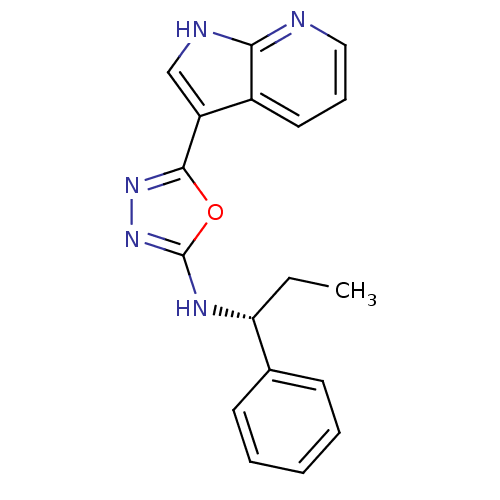
(CHEMBL3093072)Show SMILES CC[C@@H](Nc1nnc(o1)-c1c[nH]c2ncccc12)c1ccccc1 |r| Show InChI InChI=1S/C18H17N5O/c1-2-15(12-7-4-3-5-8-12)21-18-23-22-17(24-18)14-11-20-16-13(14)9-6-10-19-16/h3-11,15H,2H2,1H3,(H,19,20)(H,21,23)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cdc7/Dbf4 (unknown origin)-mediated MCM2 phosphorylation at Ser53 by protein A amplified luminescent proximity homogeneous assay |
Bioorg Med Chem Lett 23: 6396-400 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.055
BindingDB Entry DOI: 10.7270/Q2P84DC0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318880
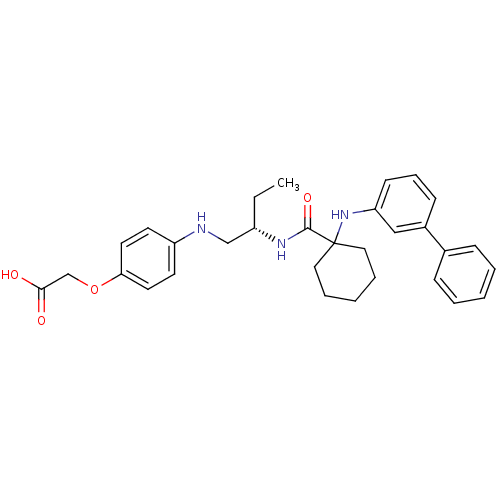
((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318880

((S)-2-(4-(2-(1-(biphenyl-3-ylamino)cyclohexanecarb...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50312922
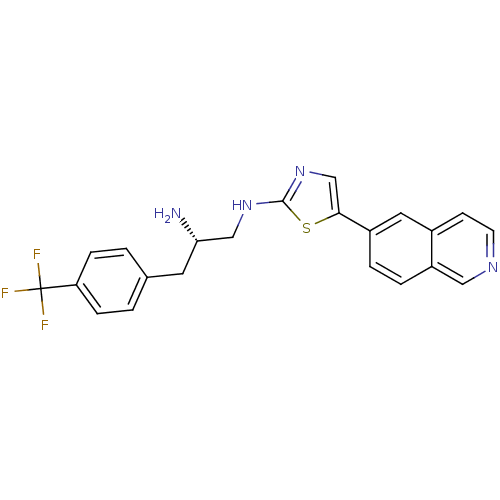
((S)-N1-(5-(isoquinolin-6-yl)thiazol-2-yl)-3-(4-(tr...)Show SMILES N[C@H](CNc1ncc(s1)-c1ccc2cnccc2c1)Cc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C22H19F3N4S/c23-22(24,25)18-5-1-14(2-6-18)9-19(26)12-28-21-29-13-20(30-21)16-3-4-17-11-27-8-7-15(17)10-16/h1-8,10-11,13,19H,9,12,26H2,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 in human U87MG cells assessed as PRAS40 phosphorylation after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1559-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.067
BindingDB Entry DOI: 10.7270/Q20C4VWX |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50446517

(CHEMBL3110092)Show SMILES CCOc1ccc(N2CCN([C@@H](C)C2)c2noc(n2)[C@H](C)NC(C)=O)c(C)c1 |r| Show InChI InChI=1S/C20H29N5O3/c1-6-27-17-7-8-18(13(2)11-17)24-9-10-25(14(3)12-24)20-22-19(28-23-20)15(4)21-16(5)26/h7-8,11,14-15H,6,9-10,12H2,1-5H3,(H,21,26)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 expressed in CHOK1 cells assessed as acetylCoA to malonylCoA conversion after 1 hr by LC-MS/MS analysis |
J Med Chem 56: 10132-41 (2013)
Article DOI: 10.1021/jm401601s
BindingDB Entry DOI: 10.7270/Q2WW7K5K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data