 Found 1093 hits with Last Name = 'allen' and Initial = 'm'
Found 1093 hits with Last Name = 'allen' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
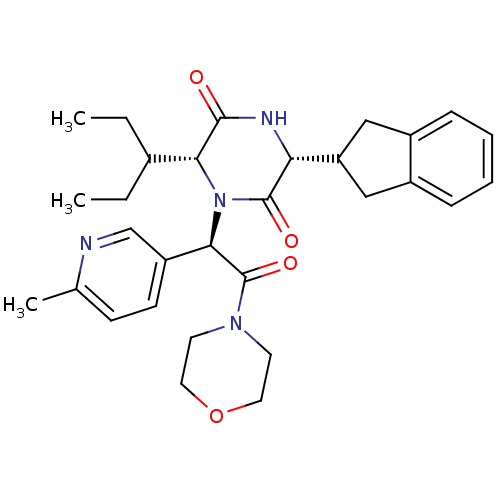
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
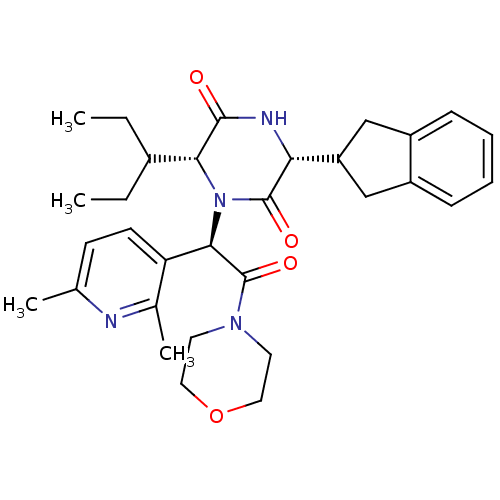
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
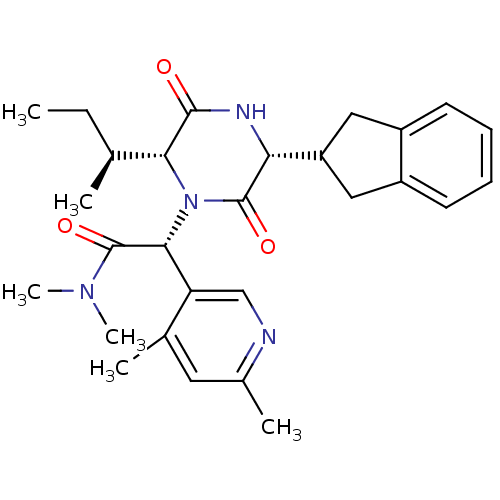
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
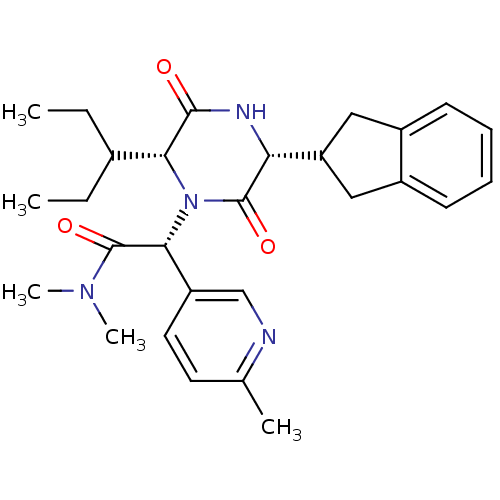
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
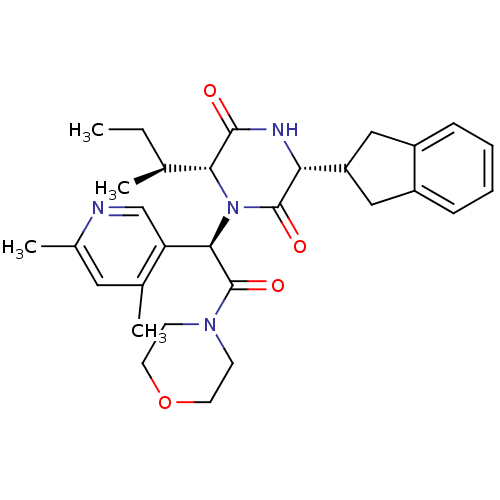
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
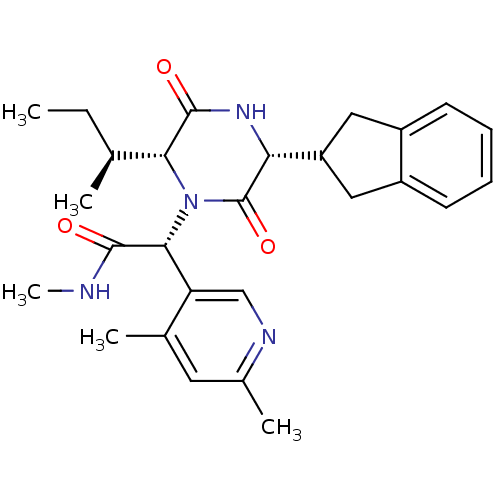
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
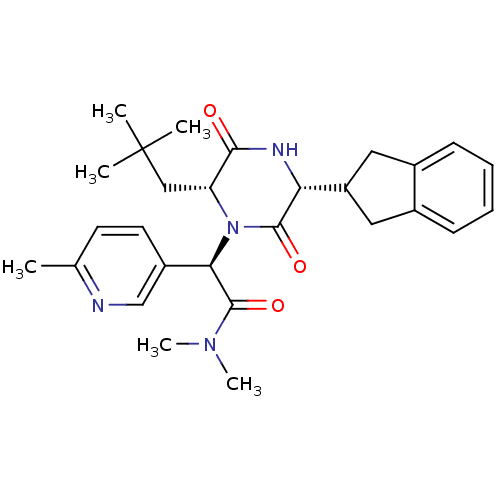
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
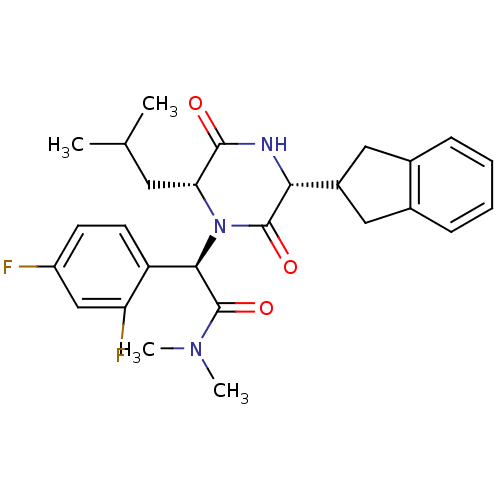
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
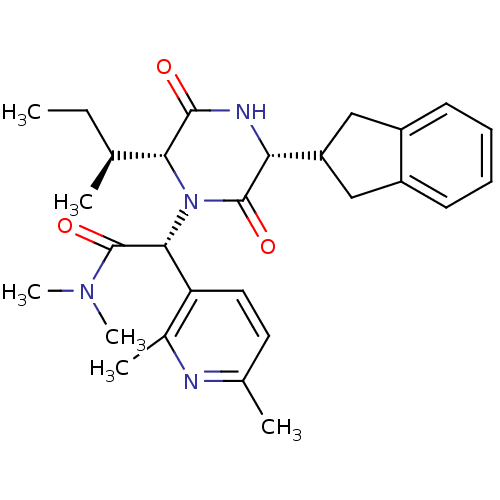
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
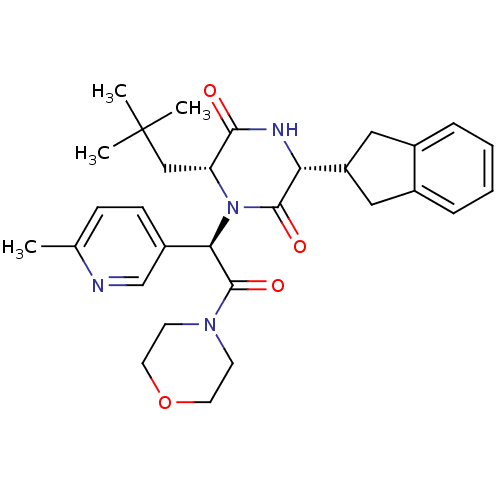
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
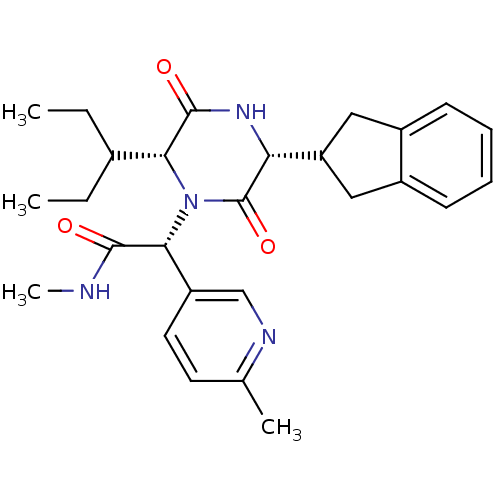
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
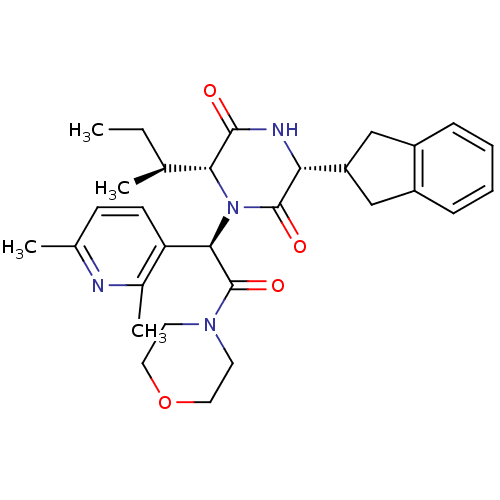
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
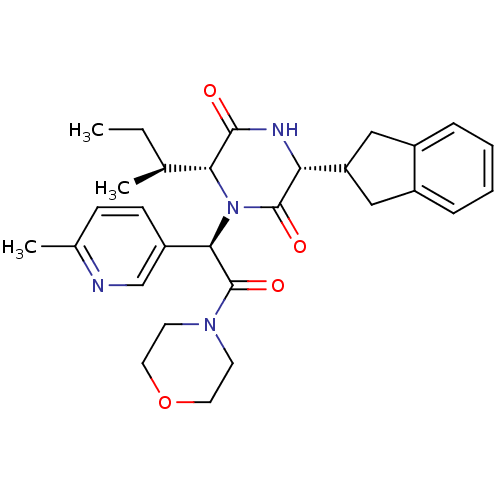
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
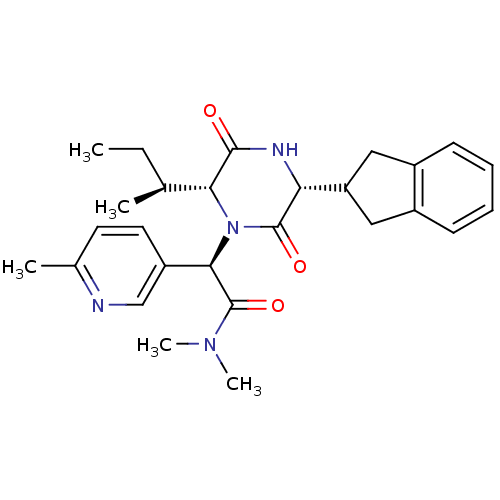
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
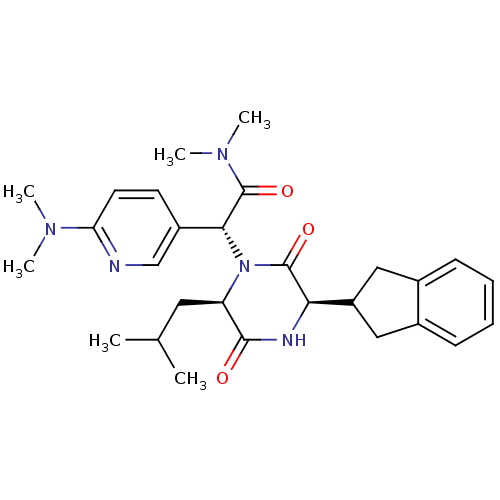
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384836
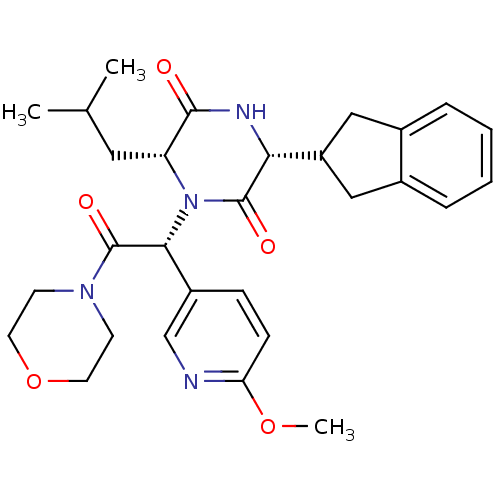
(CHEMBL2037487)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C29H36N4O5/c1-18(2)14-23-27(34)31-25(22-15-19-6-4-5-7-20(19)16-22)28(35)33(23)26(21-8-9-24(37-3)30-17-21)29(36)32-10-12-38-13-11-32/h4-9,17-18,22-23,25-26H,10-16H2,1-3H3,(H,31,34)/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384811
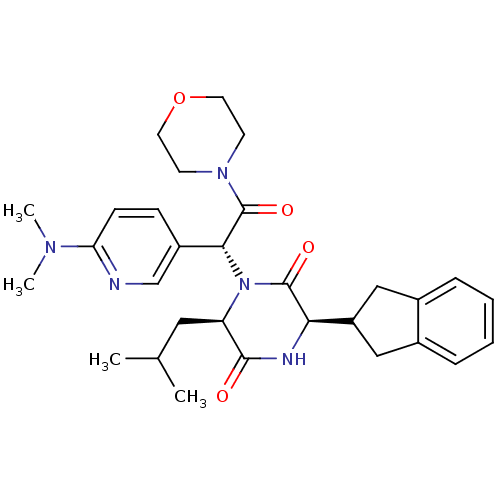
(CHEMBL2037490)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H39N5O4/c1-19(2)15-24-28(36)32-26(23-16-20-7-5-6-8-21(20)17-23)29(37)35(24)27(30(38)34-11-13-39-14-12-34)22-9-10-25(31-18-22)33(3)4/h5-10,18-19,23-24,26-27H,11-17H2,1-4H3,(H,32,36)/t24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384821
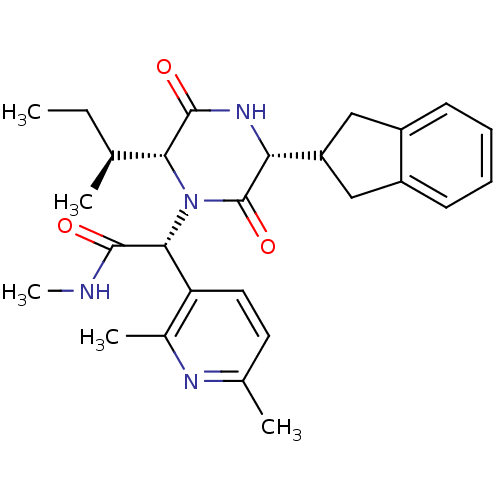
(CHEMBL2037509)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-13-18-9-7-8-10-19(18)14-20)27(34)31(23)24(25(32)28-5)21-12-11-16(3)29-17(21)4/h7-12,15,20,22-24H,6,13-14H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384835
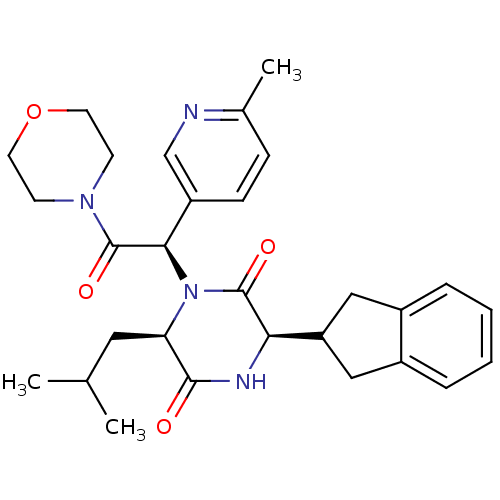
(CHEMBL2037492)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)14-24-27(34)31-25(23-15-20-6-4-5-7-21(20)16-23)28(35)33(24)26(22-9-8-19(3)30-17-22)29(36)32-10-12-37-13-11-32/h4-9,17-18,23-26H,10-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384814
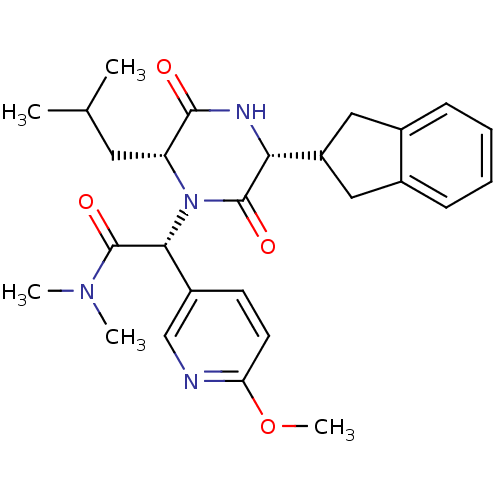
(CHEMBL2037486)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N(C)C |r| Show InChI InChI=1S/C27H34N4O4/c1-16(2)12-21-25(32)29-23(20-13-17-8-6-7-9-18(17)14-20)26(33)31(21)24(27(34)30(3)4)19-10-11-22(35-5)28-15-19/h6-11,15-16,20-21,23-24H,12-14H2,1-5H3,(H,29,32)/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441
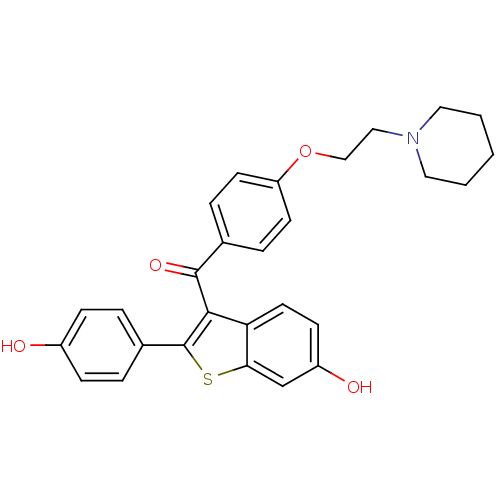
(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384809

(CHEMBL2037493)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O3/c1-18(2)14-24-27(34)31-25(23-15-20-8-4-5-9-21(20)16-23)28(35)33(24)26(22-11-10-19(3)30-17-22)29(36)32-12-6-7-13-32/h4-5,8-11,17-18,23-26H,6-7,12-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384804
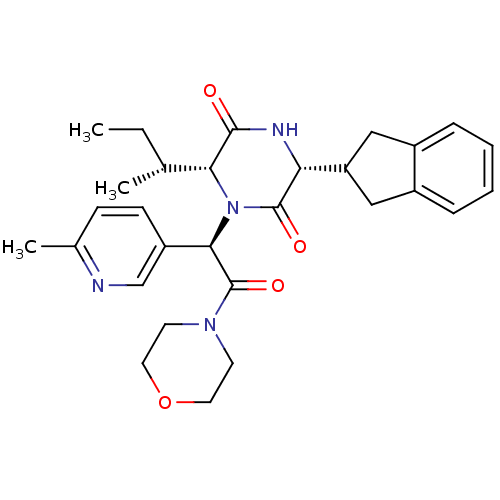
(CHEMBL2037500)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410477

(CHEMBL54719)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(OCc4ccccn4)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-14-10-15-7-9-29(19(15)11-18(14)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-13-17-4-2-3-8-26-17/h2-6,8,10-12H,7,9,13H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219760
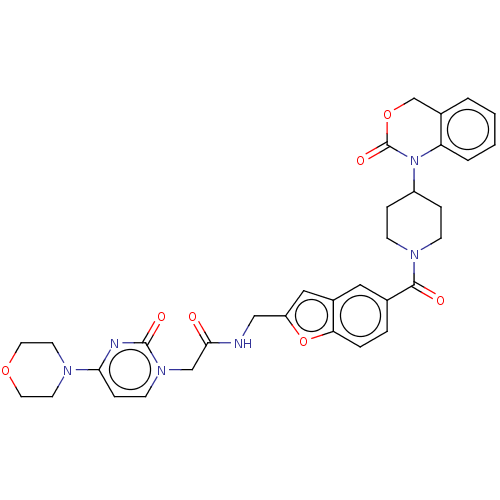
(CHEMBL286895)Show SMILES O=C(Cn1ccc(nc1=O)N1CCOCC1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C33H34N6O7/c40-30(20-38-12-9-29(35-32(38)42)36-13-15-44-16-14-36)34-19-26-18-24-17-22(5-6-28(24)46-26)31(41)37-10-7-25(8-11-37)39-27-4-2-1-3-23(27)21-45-33(39)43/h1-6,9,12,17-18,25H,7-8,10-11,13-16,19-21H2,(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410476
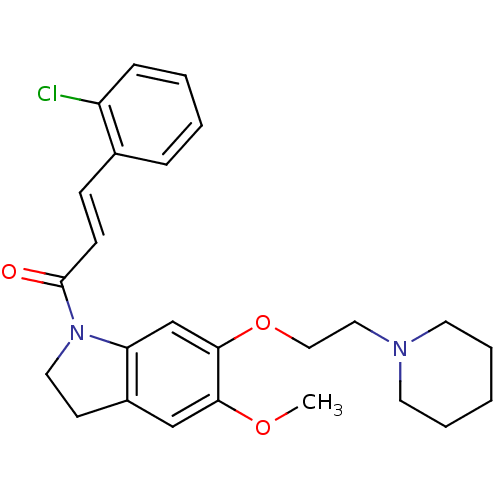
(CHEMBL197630)Show SMILES COc1cc2CCN(C(=O)\C=C\c3ccccc3Cl)c2cc1OCCN1CCCCC1 Show InChI InChI=1S/C25H29ClN2O3/c1-30-23-17-20-11-14-28(25(29)10-9-19-7-3-4-8-21(19)26)22(20)18-24(23)31-16-15-27-12-5-2-6-13-27/h3-4,7-10,17-18H,2,5-6,11-16H2,1H3/b10-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384839
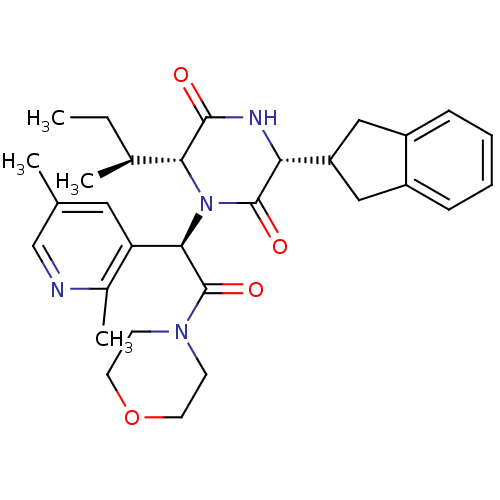
(CHEMBL2037505)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-19(3)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-14-18(2)17-31-20(24)4)30(37)33-10-12-38-13-11-33/h6-9,14,17,19,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t19-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410480
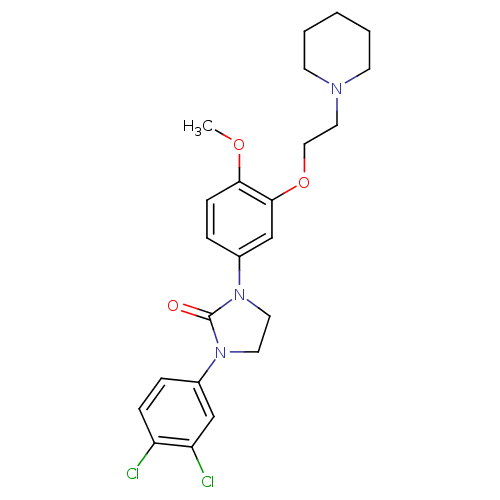
(CHEMBL197807)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C23H27Cl2N3O3/c1-30-21-8-6-18(16-22(21)31-14-13-26-9-3-2-4-10-26)28-12-11-27(23(28)29)17-5-7-19(24)20(25)15-17/h5-8,15-16H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384801

(CHEMBL2037504)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-17(3)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-12-16(2)15-29-18(22)4/h8-12,15,17,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t17-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50306288
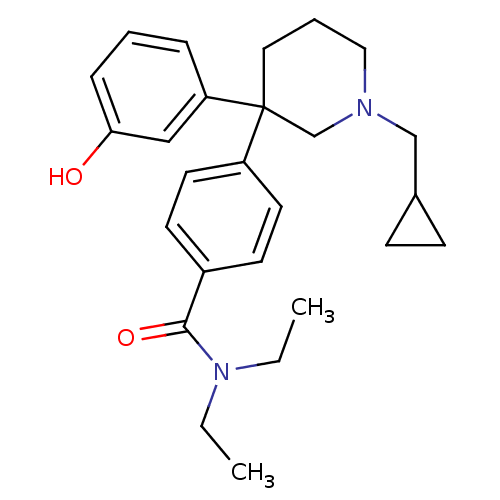
(4-(1-(cyclopropylmethyl)-3-(3-hydroxyphenyl)piperi...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1(CCCN(CC2CC2)C1)c1cccc(O)c1 Show InChI InChI=1S/C26H34N2O2/c1-3-28(4-2)25(30)21-11-13-22(14-12-21)26(23-7-5-8-24(29)17-23)15-6-16-27(19-26)18-20-9-10-20/h5,7-8,11-14,17,20,29H,3-4,6,9-10,15-16,18-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting |
Bioorg Med Chem Lett 20: 503-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.113
BindingDB Entry DOI: 10.7270/Q2C53KX1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50306298
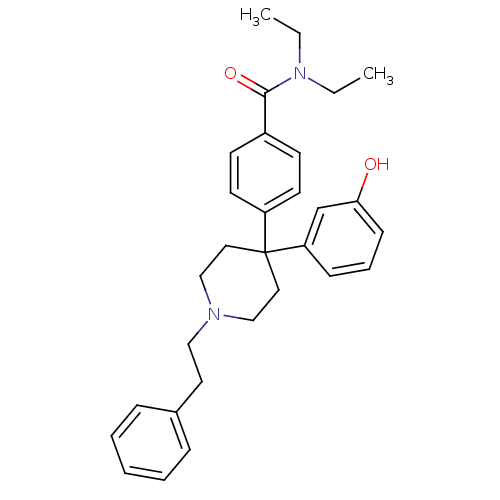
(CHEMBL595472 | N,N-diethyl-4-(4-(3-hydroxyphenyl)-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1(CCN(CCc2ccccc2)CC1)c1cccc(O)c1 Show InChI InChI=1S/C30H36N2O2/c1-3-32(4-2)29(34)25-13-15-26(16-14-25)30(27-11-8-12-28(33)23-27)18-21-31(22-19-30)20-17-24-9-6-5-7-10-24/h5-16,23,33H,3-4,17-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting |
Bioorg Med Chem Lett 20: 503-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.113
BindingDB Entry DOI: 10.7270/Q2C53KX1 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384833
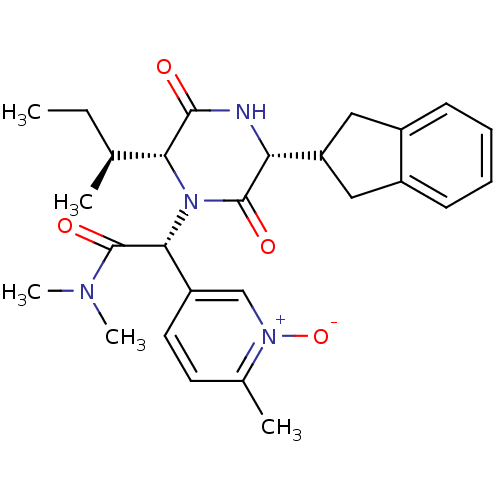
(CHEMBL2037502)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)[n+]([O-])c2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O4/c1-6-16(2)23-25(32)28-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)29(4)5)20-12-11-17(3)30(35)15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,28,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086065

(5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-ca...)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-13-10-15-7-9-29(18(15)11-17(13)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-19-4-3-8-26-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
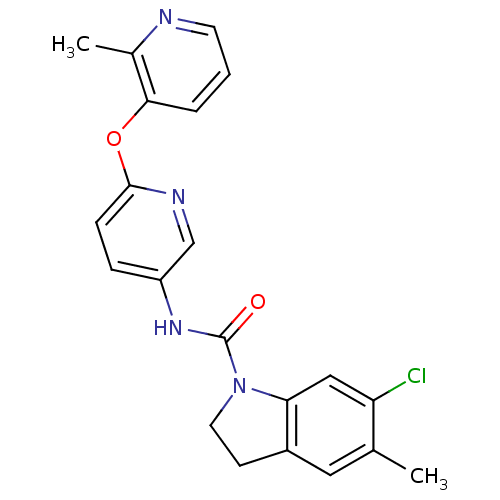
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410475
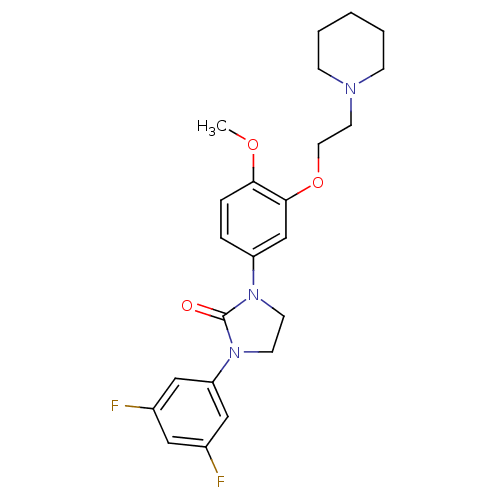
(CHEMBL372339)Show SMILES COc1ccc(cc1OCCN1CCCCC1)N1CCN(C1=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C23H27F2N3O3/c1-30-21-6-5-19(16-22(21)31-12-11-26-7-3-2-4-8-26)27-9-10-28(23(27)29)20-14-17(24)13-18(25)15-20/h5-6,13-16H,2-4,7-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384806

(CHEMBL2037498)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50372608
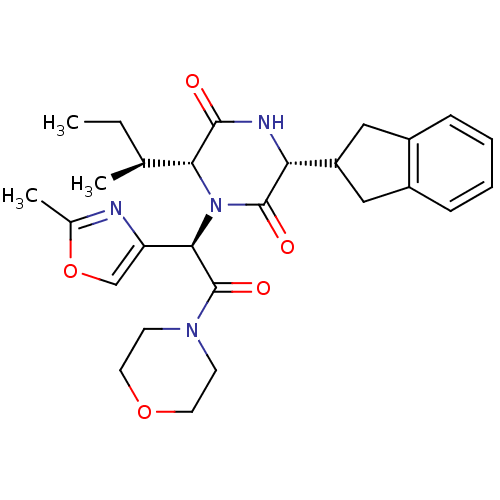
(CHEMBL429736 | GSK-221149A)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2coc(C)n2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H34N4O5/c1-4-16(2)23-25(32)29-22(20-13-18-7-5-6-8-19(18)14-20)26(33)31(23)24(21-15-36-17(3)28-21)27(34)30-9-11-35-12-10-30/h5-8,15-16,20,22-24H,4,9-14H2,1-3H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human oxytocin receptor |
Bioorg Med Chem Lett 18: 90-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.008
BindingDB Entry DOI: 10.7270/Q2XD12H2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219779
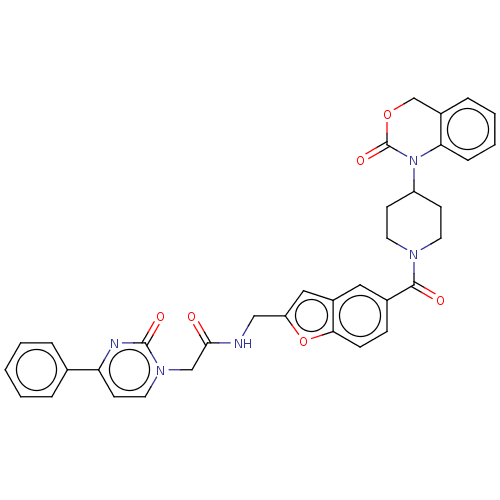
(CHEMBL30510)Show SMILES O=C(Cn1ccc(nc1=O)-c1ccccc1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C35H31N5O6/c41-32(21-39-17-14-29(37-34(39)43)23-6-2-1-3-7-23)36-20-28-19-26-18-24(10-11-31(26)46-28)33(42)38-15-12-27(13-16-38)40-30-9-5-4-8-25(30)22-45-35(40)44/h1-11,14,17-19,27H,12-13,15-16,20-22H2,(H,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788
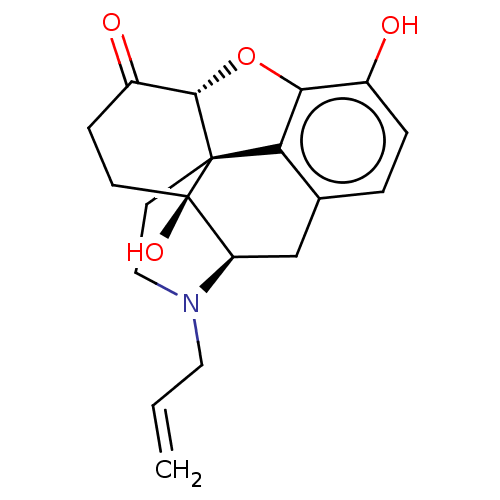
((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells |
Bioorg Med Chem Lett 10: 523-6 (2000)
BindingDB Entry DOI: 10.7270/Q2BG2N79 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50086359
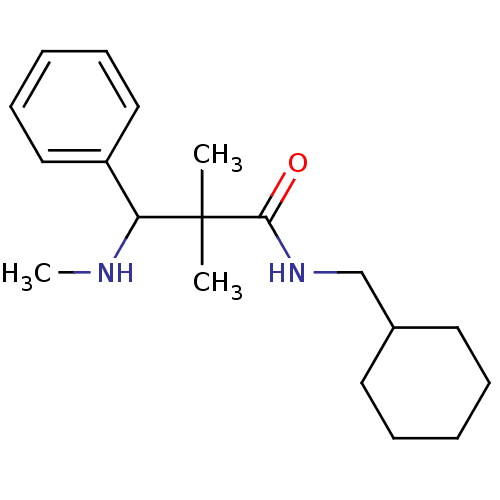
(CHEMBL147191 | N-Cyclohexylmethyl-2,2-dimethyl-3-m...)Show InChI InChI=1S/C19H30N2O/c1-19(2,17(20-3)16-12-8-5-9-13-16)18(22)21-14-15-10-6-4-7-11-15/h5,8-9,12-13,15,17,20H,4,6-7,10-11,14H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells |
Bioorg Med Chem Lett 10: 523-6 (2000)
BindingDB Entry DOI: 10.7270/Q2BG2N79 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50306293
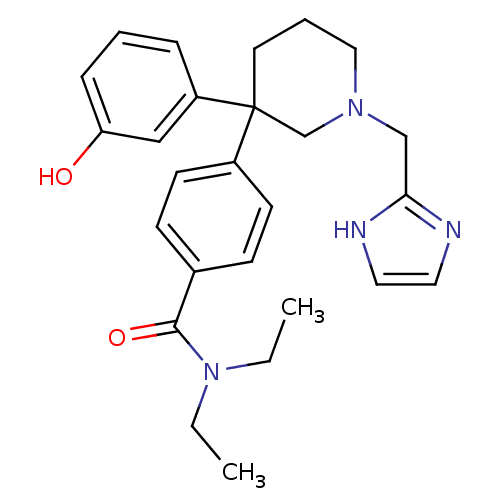
(4-(1-((1H-imidazol-2-yl)methyl)-3-(3-hydroxyphenyl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1(CCCN(Cc2ncc[nH]2)C1)c1cccc(O)c1 Show InChI InChI=1S/C26H32N4O2/c1-3-30(4-2)25(32)20-9-11-21(12-10-20)26(22-7-5-8-23(31)17-22)13-6-16-29(19-26)18-24-27-14-15-28-24/h5,7-12,14-15,17,31H,3-4,6,13,16,18-19H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting |
Bioorg Med Chem Lett 20: 503-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.113
BindingDB Entry DOI: 10.7270/Q2C53KX1 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384802

(CHEMBL2037503)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(3)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-11-15(2)14-29-17(21)4/h7-11,14,16,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410478
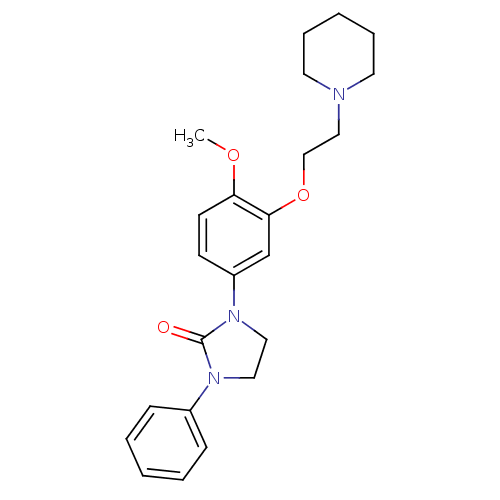
(CHEMBL383800)Show InChI InChI=1S/C23H29N3O3/c1-28-21-11-10-20(18-22(21)29-17-16-24-12-6-3-7-13-24)26-15-14-25(23(26)27)19-8-4-2-5-9-19/h2,4-5,8-11,18H,3,6-7,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410467
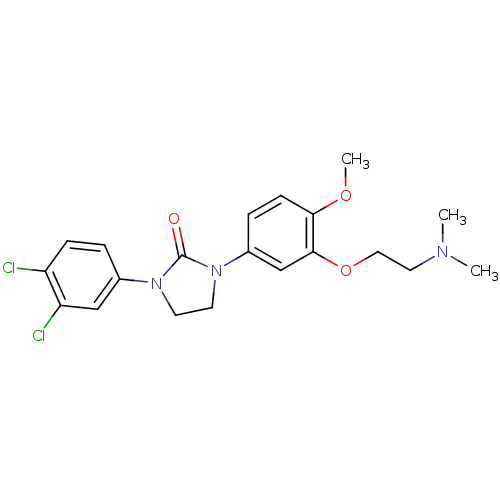
(CHEMBL196953)Show SMILES COc1ccc(cc1OCCN(C)C)N1CCN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H23Cl2N3O3/c1-23(2)10-11-28-19-13-15(5-7-18(19)27-3)25-9-8-24(20(25)26)14-4-6-16(21)17(22)12-14/h4-7,12-13H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50086361

(3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...)Show SMILES CNC(c1cccc(O)c1)C(C)(C)C(=O)NCc1cccc2ccccc12 Show InChI InChI=1S/C23H26N2O2/c1-23(2,21(24-3)17-10-7-12-19(26)14-17)22(27)25-15-18-11-6-9-16-8-4-5-13-20(16)18/h4-14,21,24,26H,15H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells |
Bioorg Med Chem Lett 10: 523-6 (2000)
BindingDB Entry DOI: 10.7270/Q2BG2N79 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219786
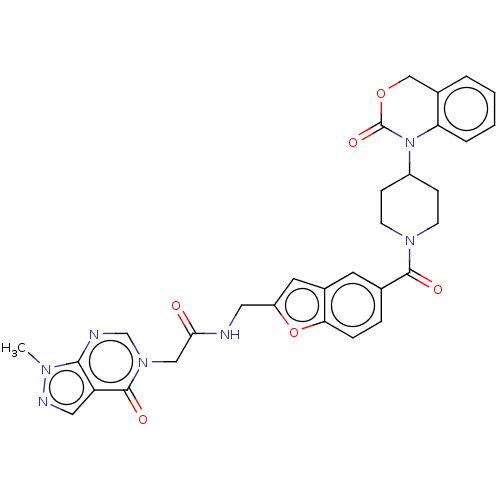
(CHEMBL285820)Show SMILES Cn1ncc2c1ncn(CC(=O)NCc1cc3cc(ccc3o1)C(=O)N1CCC(CC1)N1C(=O)OCc3ccccc13)c2=O Show InChI InChI=1S/C31H29N7O6/c1-35-28-24(15-34-35)30(41)37(18-33-28)16-27(39)32-14-23-13-21-12-19(6-7-26(21)44-23)29(40)36-10-8-22(9-11-36)38-25-5-3-2-4-20(25)17-43-31(38)42/h2-7,12-13,15,18,22H,8-11,14,16-17H2,1H3,(H,32,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50086367
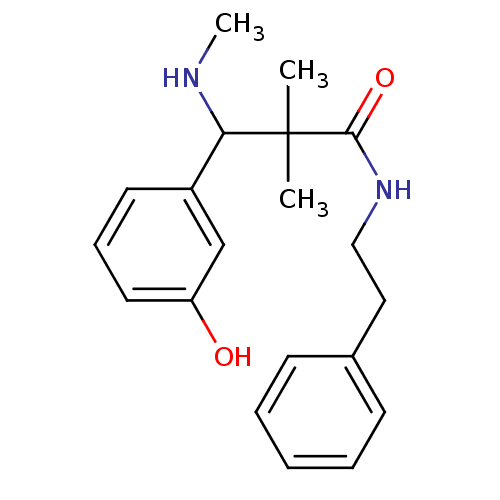
(3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...)Show InChI InChI=1S/C20H26N2O2/c1-20(2,18(21-3)16-10-7-11-17(23)14-16)19(24)22-13-12-15-8-5-4-6-9-15/h4-11,14,18,21,23H,12-13H2,1-3H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAMGO binding to rat Opioid receptor mu 1 |
Bioorg Med Chem Lett 10: 523-6 (2000)
BindingDB Entry DOI: 10.7270/Q2BG2N79 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50086361

(3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...)Show SMILES CNC(c1cccc(O)c1)C(C)(C)C(=O)NCc1cccc2ccccc12 Show InChI InChI=1S/C23H26N2O2/c1-23(2,21(24-3)17-10-7-12-19(26)14-17)22(27)25-15-18-11-6-9-16-8-4-5-13-20(16)18/h4-14,21,24,26H,15H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAMGO binding to rat Opioid receptor mu 1 |
Bioorg Med Chem Lett 10: 523-6 (2000)
BindingDB Entry DOI: 10.7270/Q2BG2N79 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data