 Found 13500 hits with Last Name = 'allen' and Initial = 's'
Found 13500 hits with Last Name = 'allen' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604668
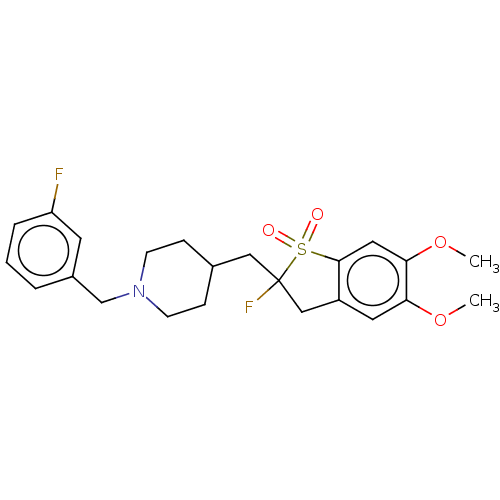
(CHEMBL5180947)Show SMILES COc1cc2CC(F)(CC3CCN(Cc4cccc(F)c4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312651
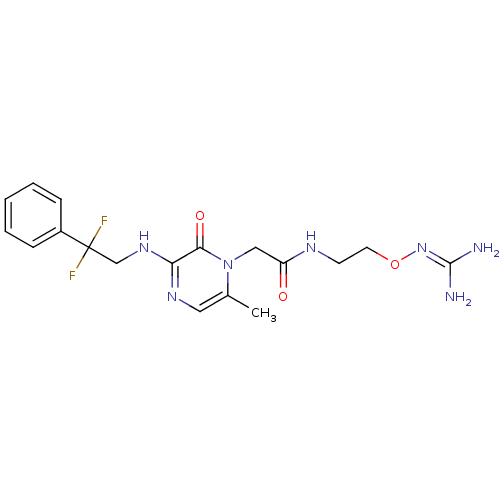
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C18H23F2N7O3/c1-12-9-24-15(25-11-18(19,20)13-5-3-2-4-6-13)16(29)27(12)10-14(28)23-7-8-30-26-17(21)22/h2-6,9H,7-8,10-11H2,1H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
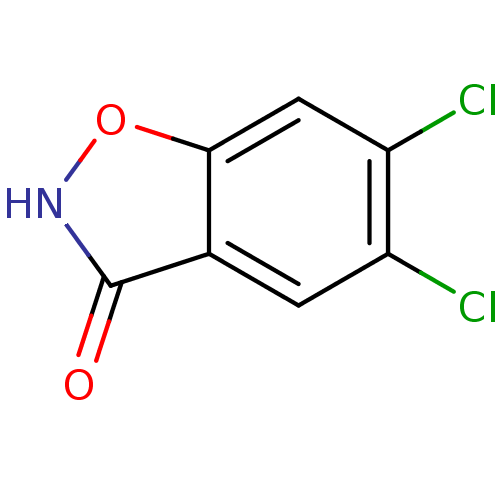
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312653
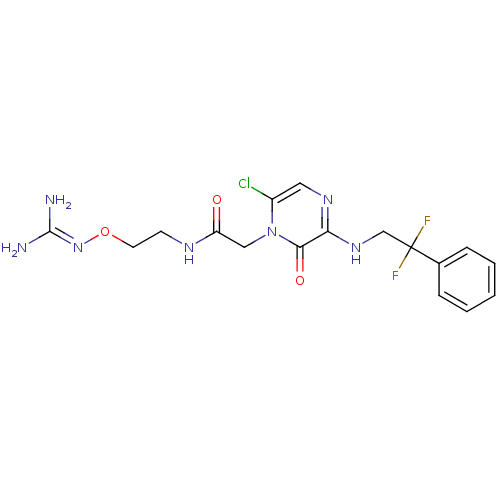
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-n1c(Cl)cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c1=O Show InChI InChI=1S/C17H20ClF2N7O3/c18-12-8-24-14(25-10-17(19,20)11-4-2-1-3-5-11)15(29)27(12)9-13(28)23-6-7-30-26-16(21)22/h1-5,8H,6-7,9-10H2,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312652
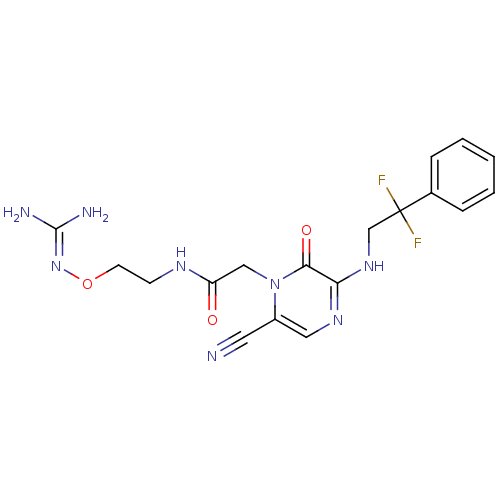
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-n1c(cnc(-[#7]-[#6]C(F)(F)c2ccccc2)c1=O)C#N Show InChI InChI=1S/C18H20F2N8O3/c19-18(20,12-4-2-1-3-5-12)11-26-15-16(30)28(13(8-21)9-25-15)10-14(29)24-6-7-31-27-17(22)23/h1-5,9H,6-7,10-11H2,(H,24,29)(H,25,26)(H4,22,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
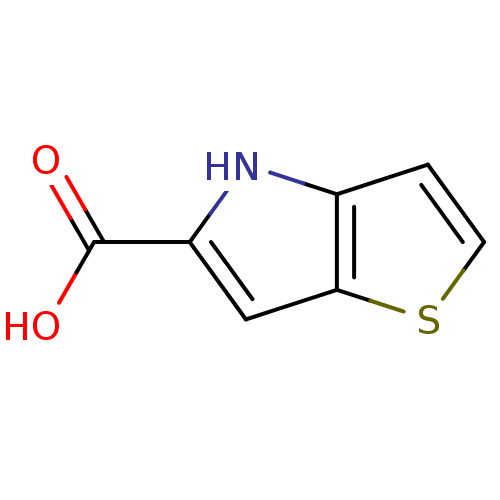
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50312663
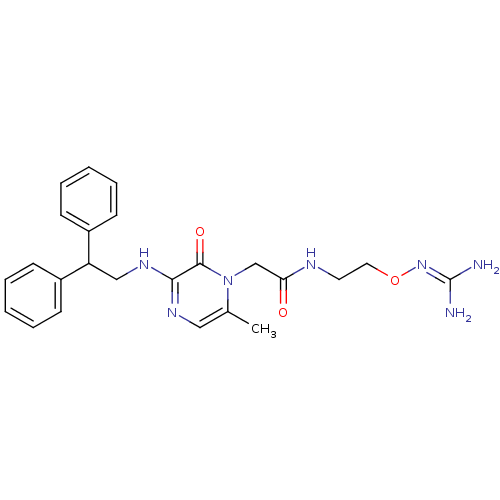
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6](-c2ccccc2)-c2ccccc2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C24H29N7O3/c1-17-14-28-22(23(33)31(17)16-21(32)27-12-13-34-30-24(25)26)29-15-20(18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-11,14,20H,12-13,15-16H2,1H3,(H,27,32)(H,28,29)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
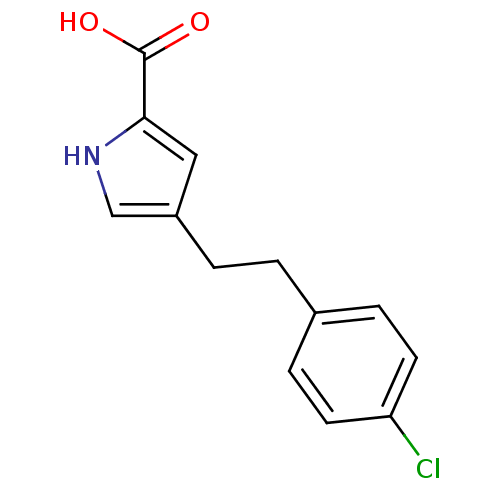
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182163
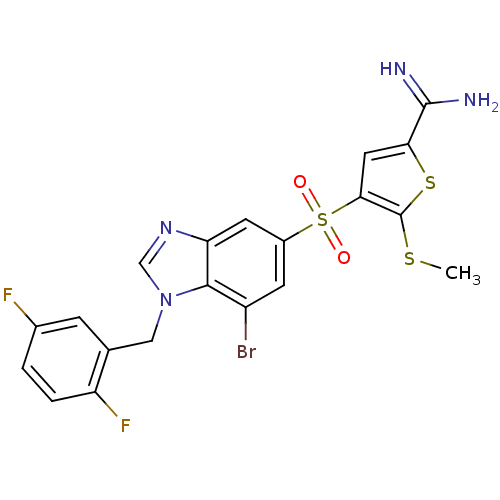
(4-[7-bromo-1-(2,5-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(F)ccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)26-9-27(18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312658
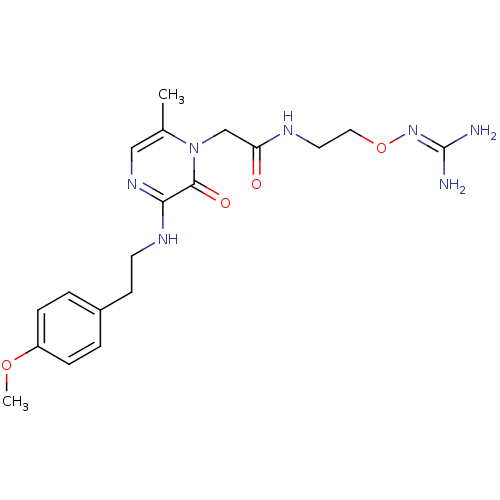
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#7]-c2ncc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c2=O)cc1 Show InChI InChI=1S/C19H27N7O4/c1-13-11-24-17(23-8-7-14-3-5-15(29-2)6-4-14)18(28)26(13)12-16(27)22-9-10-30-25-19(20)21/h3-6,11H,7-10,12H2,1-2H3,(H,22,27)(H,23,24)(H4,20,21,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312662
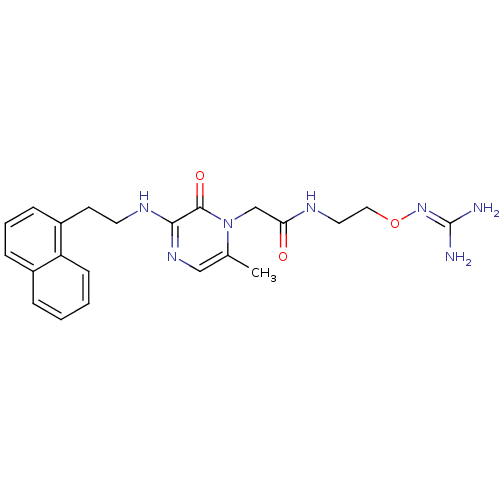
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2cccc3ccccc23)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C22H27N7O3/c1-15-13-27-20(21(31)29(15)14-19(30)25-11-12-32-28-22(23)24)26-10-9-17-7-4-6-16-5-2-3-8-18(16)17/h2-8,13H,9-12,14H2,1H3,(H,25,30)(H,26,27)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312654
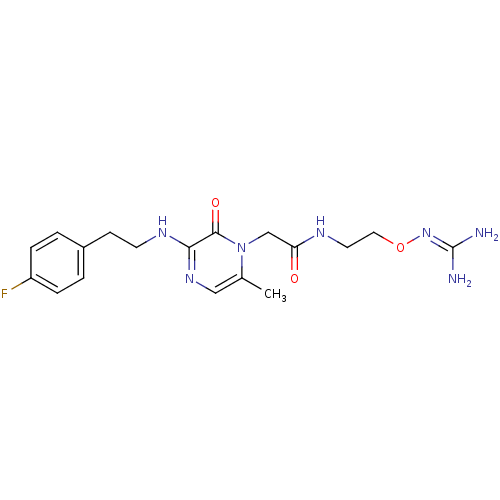
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2ccc(F)cc2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C18H24FN7O3/c1-12-10-24-16(23-7-6-13-2-4-14(19)5-3-13)17(28)26(12)11-15(27)22-8-9-29-25-18(20)21/h2-5,10H,6-9,11H2,1H3,(H,22,27)(H,23,24)(H4,20,21,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
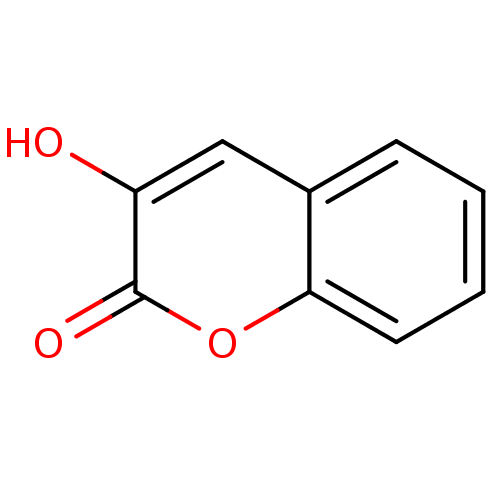
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233679
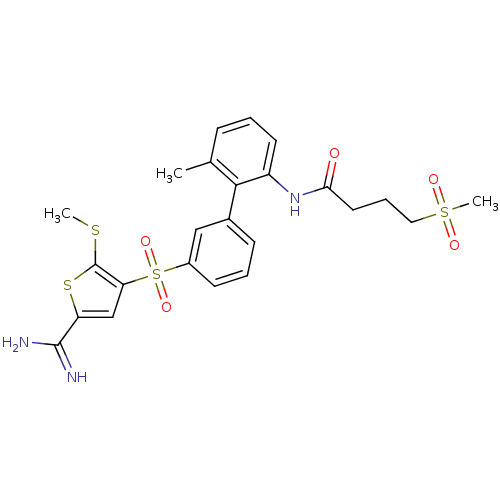
(CHEMBL399284 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C24H27N3O5S4/c1-15-7-4-10-18(27-21(28)11-6-12-35(3,29)30)22(15)16-8-5-9-17(13-16)36(31,32)20-14-19(23(25)26)34-24(20)33-2/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312656
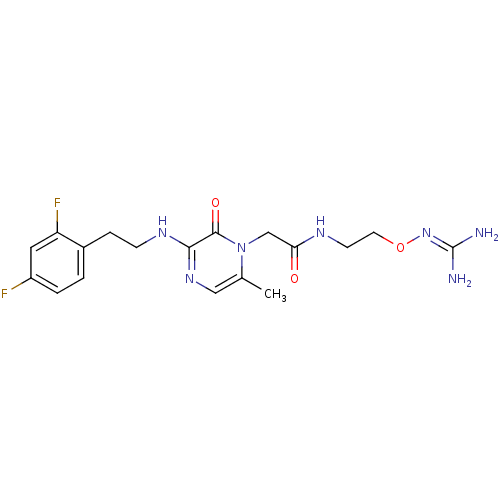
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2ccc(F)cc2F)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C18H23F2N7O3/c1-11-9-25-16(24-5-4-12-2-3-13(19)8-14(12)20)17(29)27(11)10-15(28)23-6-7-30-26-18(21)22/h2-3,8-9H,4-7,10H2,1H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182160
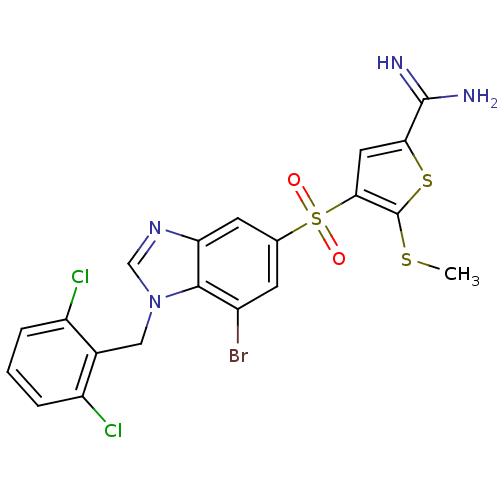
(4-[7-bromo-1-(2,6-dichloro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(Cl)cccc3Cl)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233691

(4-(2'-amino-6'-methyl-biphenyl-3-sulfonyl)-5-methy...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1N)C(N)=N Show InChI InChI=1S/C19H19N3O2S3/c1-11-5-3-8-14(20)17(11)12-6-4-7-13(9-12)27(23,24)16-10-15(18(21)22)26-19(16)25-2/h3-10H,20H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233674
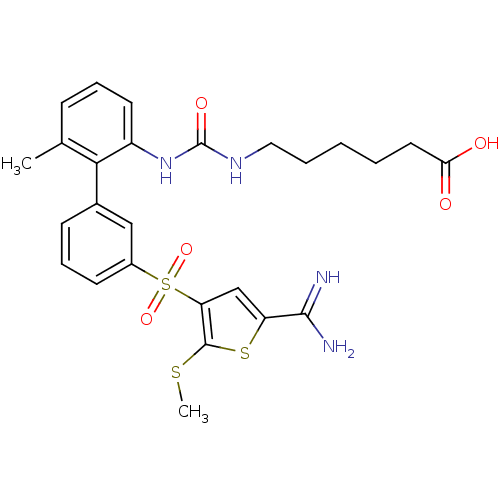
(6-{3-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophe...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCCCCC(O)=O)C(N)=N Show InChI InChI=1S/C26H30N4O5S3/c1-16-8-6-11-19(30-26(33)29-13-5-3-4-12-22(31)32)23(16)17-9-7-10-18(14-17)38(34,35)21-15-20(24(27)28)37-25(21)36-2/h6-11,14-15H,3-5,12-13H2,1-2H3,(H3,27,28)(H,31,32)(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182171
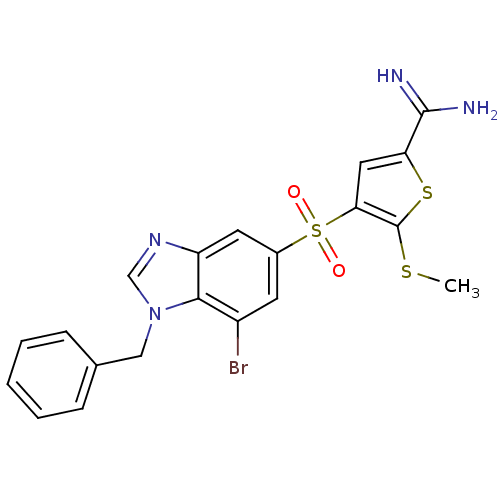
(4-(1-benzyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3ccccc3)cnc2c1)C(N)=N Show InChI InChI=1S/C20H17BrN4O2S3/c1-28-20-17(9-16(29-20)19(22)23)30(26,27)13-7-14(21)18-15(8-13)24-11-25(18)10-12-5-3-2-4-6-12/h2-9,11H,10H2,1H3,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233686

(4-(2'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-3-4-9-15(12)13-7-5-8-14(10-13)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182170
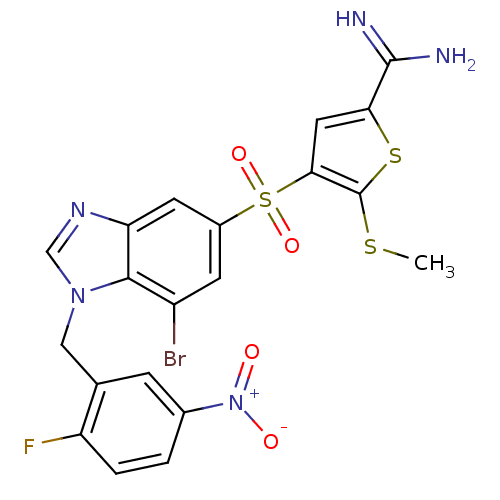
(4-[7-bromo-1-(2-fluoro-5-nitro-benzyl)-1H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(ccc3F)[N+]([O-])=O)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)25-9-26(18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312664
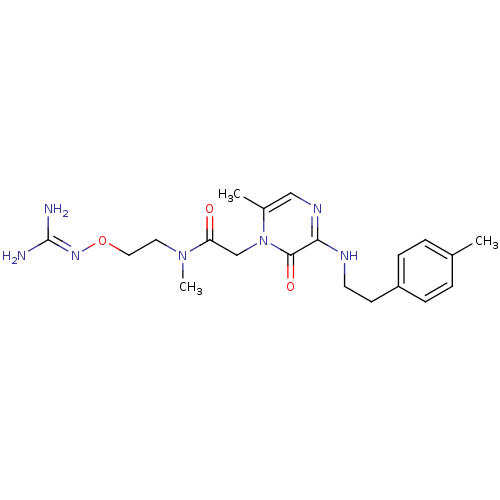
(1-{N-[2-(Amidino-N0-methylaminooxy)ethyl]amino}car...)Show SMILES [#6]-[#7](-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-n1c(-[#6])cnc(-[#7]-[#6]-[#6]-c2ccc(-[#6])cc2)c1=O Show InChI InChI=1S/C20H29N7O3/c1-14-4-6-16(7-5-14)8-9-23-18-19(29)27(15(2)12-24-18)13-17(28)26(3)10-11-30-25-20(21)22/h4-7,12H,8-11,13H2,1-3H3,(H,23,24)(H4,21,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182159
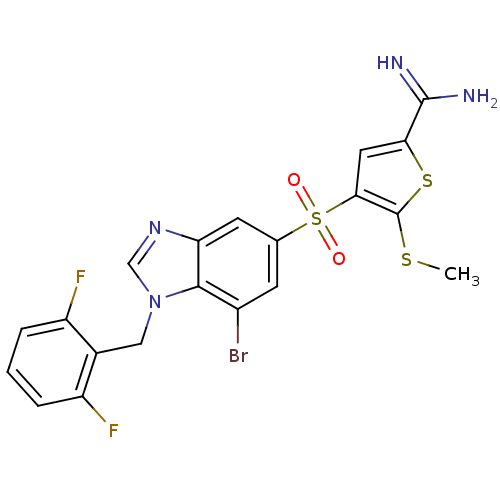
(4-[7-bromo-1-(2,6-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233692

(4-[3-(6-methyl-pyridin-2-yl)-benzenesulfonyl]-5-me...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)n1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-11-5-3-8-14(21-11)12-6-4-7-13(9-12)26(22,23)16-10-15(17(19)20)25-18(16)24-2/h3-10H,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233688

(4-(2'-chloro-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(N)=N Show InChI InChI=1S/C18H15ClN2O2S3/c1-24-18-16(10-15(25-18)17(20)21)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)19/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233694

(5-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCCC(O)=O)C(N)=N Show InChI InChI=1S/C25H27N3O5S3/c1-15-7-5-10-18(28-21(29)11-3-4-12-22(30)31)23(15)16-8-6-9-17(13-16)36(32,33)20-14-19(24(26)27)35-25(20)34-2/h5-10,13-14H,3-4,11-12H2,1-2H3,(H3,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312661
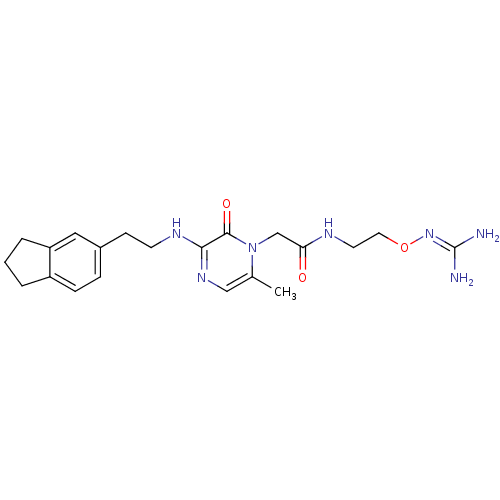
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2ccc3-[#6]-[#6]-[#6]-c3c2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C21H29N7O3/c1-14-12-26-19(25-8-7-15-5-6-16-3-2-4-17(16)11-15)20(30)28(14)13-18(29)24-9-10-31-27-21(22)23/h5-6,11-12H,2-4,7-10,13H2,1H3,(H,24,29)(H,25,26)(H4,22,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312655
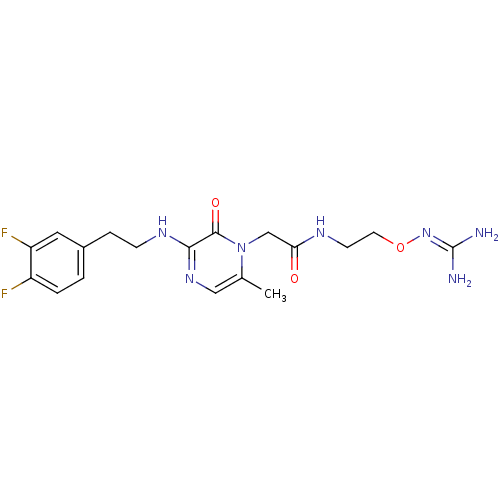
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2ccc(F)c(F)c2)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C18H23F2N7O3/c1-11-9-25-16(24-5-4-12-2-3-13(19)14(20)8-12)17(29)27(11)10-15(28)23-6-7-30-26-18(21)22/h2-3,8-9H,4-7,10H2,1H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312657
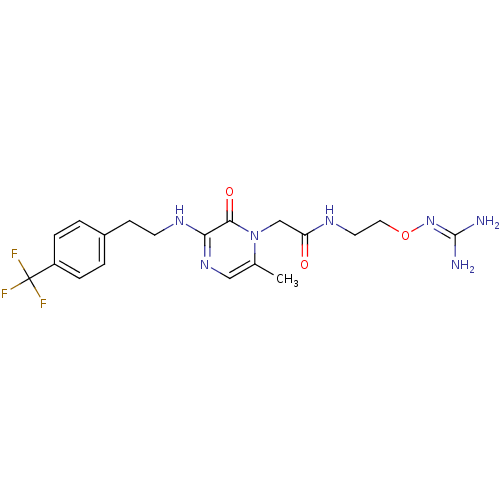
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-c1cnc(-[#7]-[#6]-[#6]-c2ccc(cc2)C(F)(F)F)c(=O)n1-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C19H24F3N7O3/c1-12-10-27-16(26-7-6-13-2-4-14(5-3-13)19(20,21)22)17(31)29(12)11-15(30)25-8-9-32-28-18(23)24/h2-5,10H,6-9,11H2,1H3,(H,25,30)(H,26,27)(H4,23,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233677

(4-(2'-hydroxymethyl-6'-methyl-biphenyl-3-sulfonyl)...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1CO)C(N)=N Show InChI InChI=1S/C20H20N2O3S3/c1-12-5-3-7-14(11-23)18(12)13-6-4-8-15(9-13)28(24,25)17-10-16(19(21)22)27-20(17)26-2/h3-10,23H,11H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182176
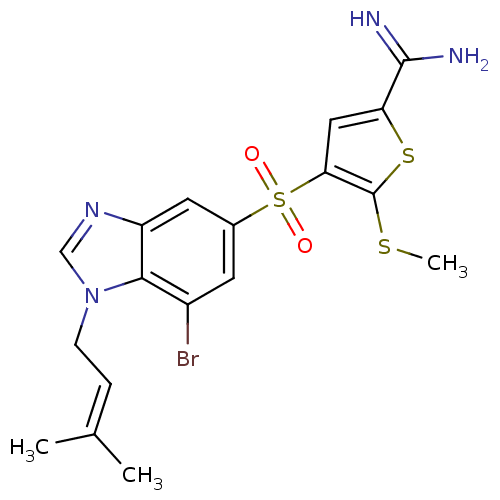
(4-[7-bromo-1-(3-methyl-but-2-enyl)-1H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2n(-[#6]\[#6]=[#6](/[#6])-[#6])cnc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-13-7-11(6-12(19)16(13)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233689
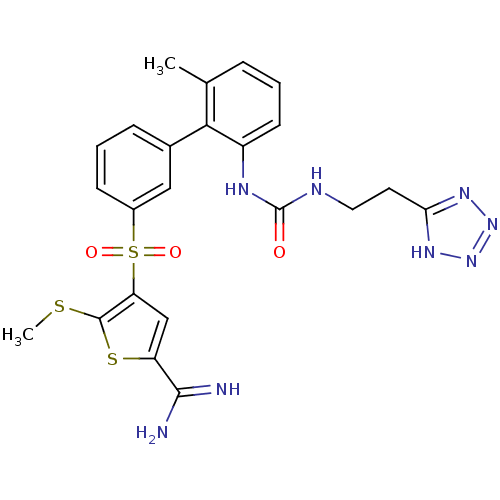
(5-methylsulfanyl-4-(6'-methyl-2'-{3-[2-(2H-tetrazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCc1nnn[nH]1)C(N)=N Show InChI InChI=1S/C23H24N8O3S3/c1-13-5-3-8-16(27-23(32)26-10-9-19-28-30-31-29-19)20(13)14-6-4-7-15(11-14)37(33,34)18-12-17(21(24)25)36-22(18)35-2/h3-8,11-12H,9-10H2,1-2H3,(H3,24,25)(H2,26,27,32)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312660
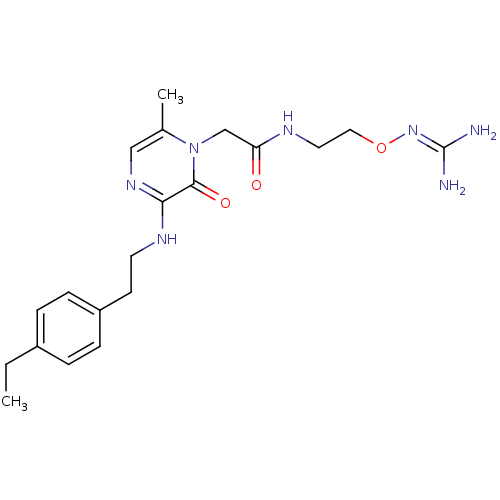
(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-[#6]-c1ccc(-[#6]-[#6]-[#7]-c2ncc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c2=O)cc1 Show InChI InChI=1S/C20H29N7O3/c1-3-15-4-6-16(7-5-15)8-9-24-18-19(29)27(14(2)12-25-18)13-17(28)23-10-11-30-26-20(21)22/h4-7,12H,3,8-11,13H2,1-2H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182185
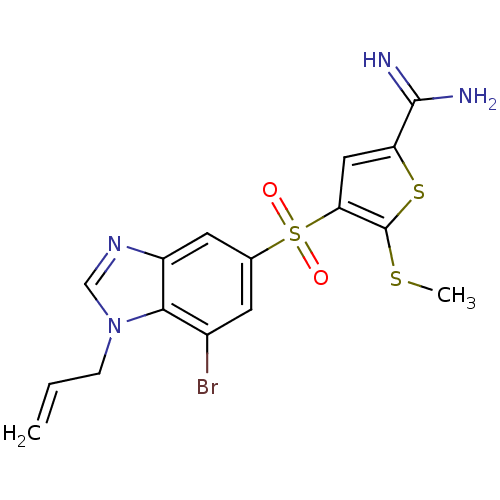
(4-(1-allyl-7-bromo-1H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(CC=C)cnc2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-11-6-9(5-10(17)14(11)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182173
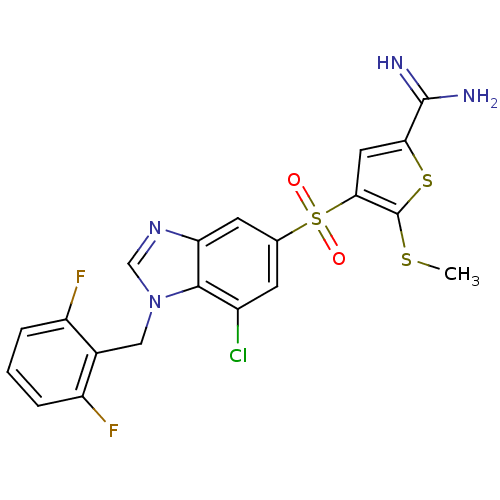
(4-[7-chloro-1-(2,6-difluoro-benzyl)-1H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2n(Cc3c(F)cccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)26-9-27(18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182183

(4-[7-chloro-3-(2,6-difluoro-benzyl)-3H-benzoimidaz...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Cl)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15ClF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233693

(CHEMBL252619 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C22H23N3O5S4/c1-13-6-4-9-16(25-19(26)12-33(3,27)28)20(13)14-7-5-8-15(10-14)34(29,30)18-11-17(21(23)24)32-22(18)31-2/h4-11H,12H2,1-3H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233680

(4-(2'-hydroxymethyl-biphenyl-3-sulfonyl)-5-methyls...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1CO)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-25-19-17(10-16(26-19)18(20)21)27(23,24)14-7-4-6-12(9-14)15-8-3-2-5-13(15)11-22/h2-10,22H,11H2,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50312659

(1-{N-[2-(Amidinoaminooxy)ethyl]amino}carbonylmethy...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#7]-c2ncc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c2=O)cc1-[#8]-[#6] Show InChI InChI=1S/C20H29N7O5/c1-13-11-25-18(24-7-6-14-4-5-15(30-2)16(10-14)31-3)19(29)27(13)12-17(28)23-8-9-32-26-20(21)22/h4-5,10-11H,6-9,12H2,1-3H3,(H,23,28)(H,24,25)(H4,21,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin |
J Med Chem 53: 1843-56 (2010)
Article DOI: 10.1021/jm901802n
BindingDB Entry DOI: 10.7270/Q22807Q9 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233681
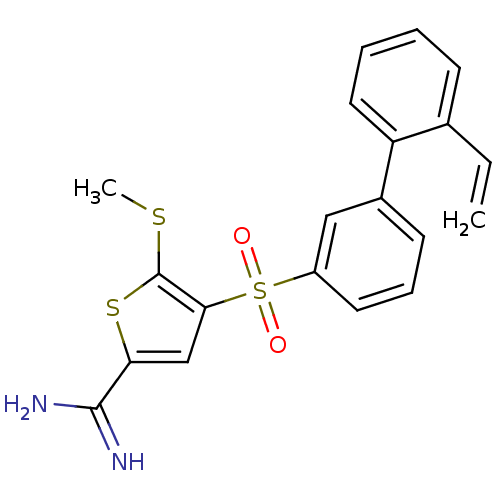
(5-methylsulfanyl-4-(2'-vinyl-biphenyl-3-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C=C)C(N)=N Show InChI InChI=1S/C20H18N2O2S3/c1-3-13-7-4-5-10-16(13)14-8-6-9-15(11-14)27(23,24)18-12-17(19(21)22)26-20(18)25-2/h3-12H,1H2,2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233683
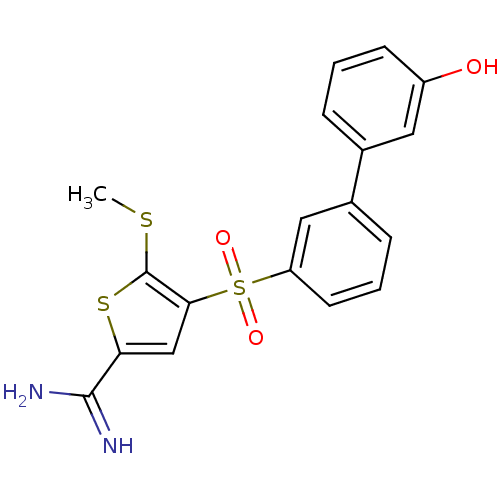
(4-(3'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(O)c1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-7-3-5-12(9-14)11-4-2-6-13(21)8-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233676

(3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-3-s...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1C(O)=O)C(N)=N Show InChI InChI=1S/C20H18N2O4S3/c1-11-5-3-8-14(19(23)24)17(11)12-6-4-7-13(9-12)29(25,26)16-10-15(18(21)22)28-20(16)27-2/h3-10H,1-2H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182164

(4-[7-bromo-3-(2,6-dichloro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(Cl)cccc3Cl)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrCl2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182181
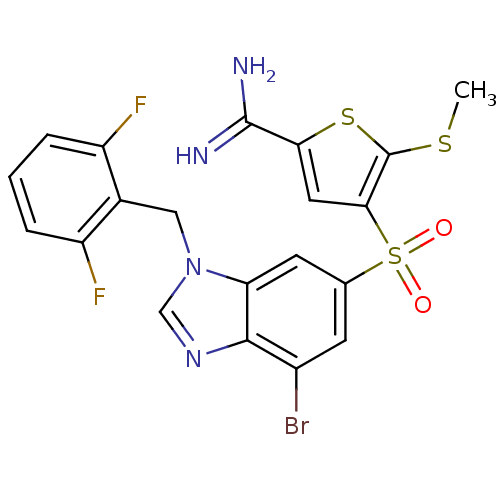
(4-[7-bromo-3-(2,6-difluoro-benzyl)-3H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3c(F)cccc3F)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)10-5-12(21)18-15(6-10)27(9-26-18)8-11-13(22)3-2-4-14(11)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182174
(4-[7-bromo-3-(2-fluoro-5-nitro-benzyl)-3H-benzoimi...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(Cc3cc(ccc3F)[N+]([O-])=O)c2c1)C(N)=N Show InChI InChI=1S/C20H15BrFN5O4S3/c1-32-20-17(7-16(33-20)19(23)24)34(30,31)12-5-13(21)18-15(6-12)26(9-25-18)8-10-4-11(27(28)29)2-3-14(10)22/h2-7,9H,8H2,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182180
(4-(7-bromo-3-phenyl-3H-benzoimidazole-5-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(-c3ccccc3)c2c1)C(N)=N Show InChI InChI=1S/C19H15BrN4O2S3/c1-27-19-16(9-15(28-19)18(21)22)29(25,26)12-7-13(20)17-14(8-12)24(10-23-17)11-5-3-2-4-6-11/h2-10H,1H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433371
(CHEMBL2375519)Show InChI InChI=1S/C15H13NO3/c17-15(18)12-8-13-14(16-12)11(9-19-13)7-6-10-4-2-1-3-5-10/h1-5,8-9,16H,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233678

(4-(4'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccc(O)cc1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-4-2-3-12(9-14)11-5-7-13(21)8-6-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182153
(4-[7-bromo-3-(3-methyl-but-2-enyl)-3H-benzoimidazo...)Show SMILES [#6]-[#16]-c1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C18H19BrN4O2S3/c1-10(2)4-5-23-9-22-16-12(19)6-11(7-13(16)23)28(24,25)15-8-14(17(20)21)27-18(15)26-3/h4,6-9H,5H2,1-3H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182182
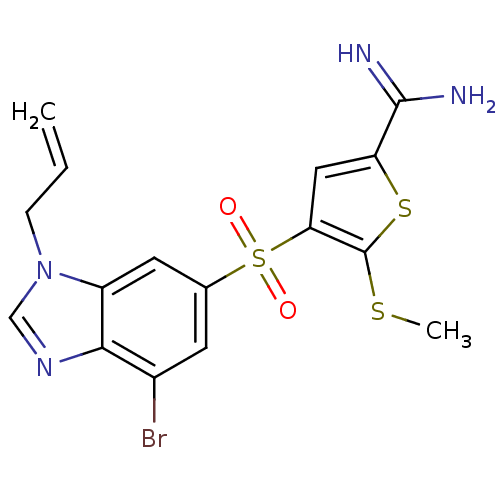
(4-(3-allyl-7-bromo-3H-benzoimidazole-5-sulfonyl)-5...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2ncn(CC=C)c2c1)C(N)=N Show InChI InChI=1S/C16H15BrN4O2S3/c1-3-4-21-8-20-14-10(17)5-9(6-11(14)21)26(22,23)13-7-12(15(18)19)25-16(13)24-2/h3,5-8H,1,4H2,2H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data