

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM166831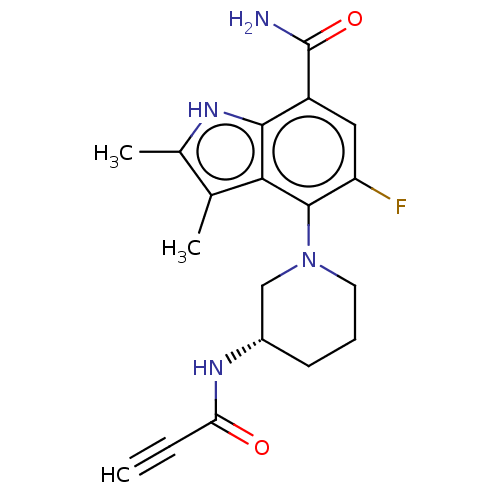 (US10604504, Example 242 | US11623921, Example 242 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165319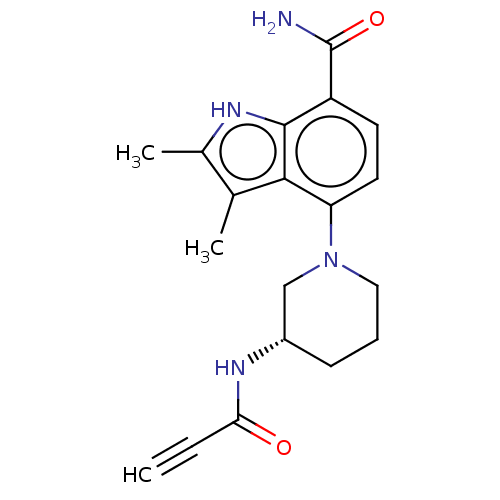 (US10604504, Example 115 | US11623921, Example 115 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC assessed as reduction in TNFalpha expression | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human peripheral B cells assessed as reduction in CD86 surface expression | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165320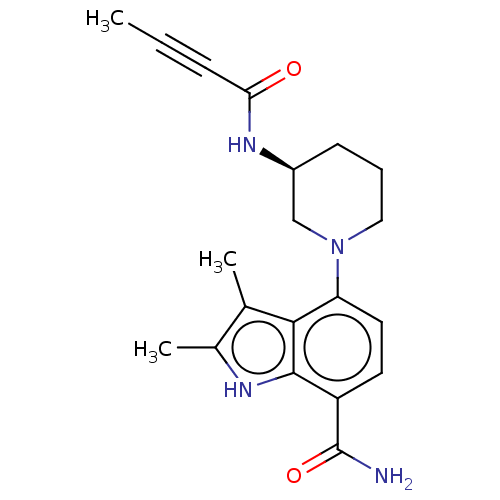 (US10604504, Example 116 | US11623921, Example 116 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165463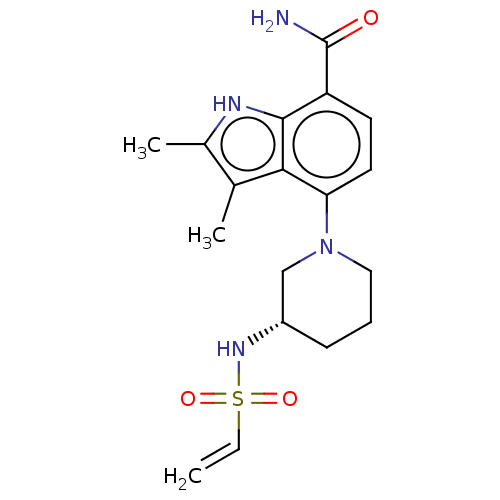 (US10604504, Example 141 | US11623921, Example 141 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165318 (US10604504, Example 114 | US11623921, Example 114 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of TEC (unknown origin) | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human memory B cells assessed as reduction in CD86 surface expression | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165465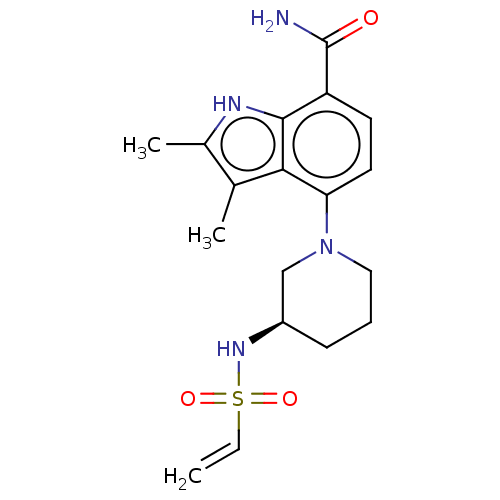 (US10604504, Example 143 | US11623921, Example 143 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165321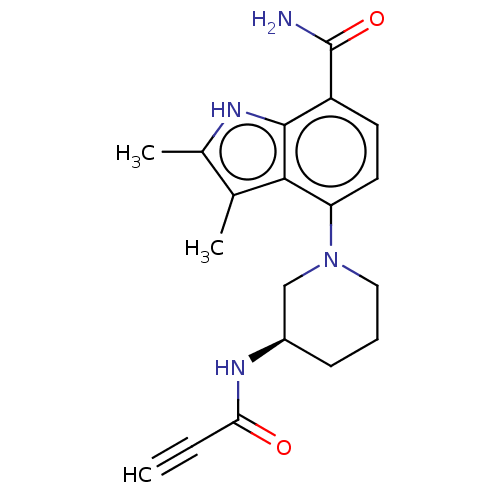 (US10604504, Example 117 | US11623921, Example 117 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165461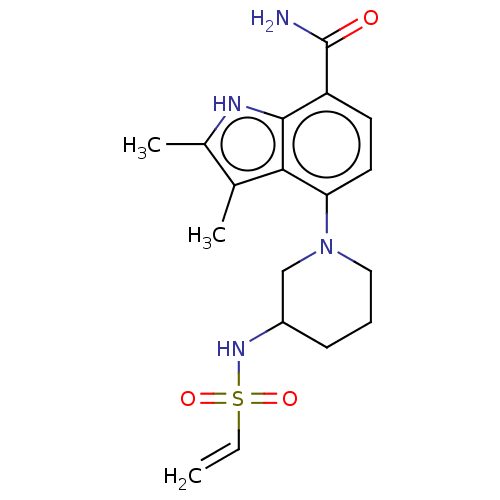 (US10604504, Example 139 | US11623921, Example 139 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BMX (unknown origin) | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM166831 (US10604504, Example 242 | US11623921, Example 242 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50540957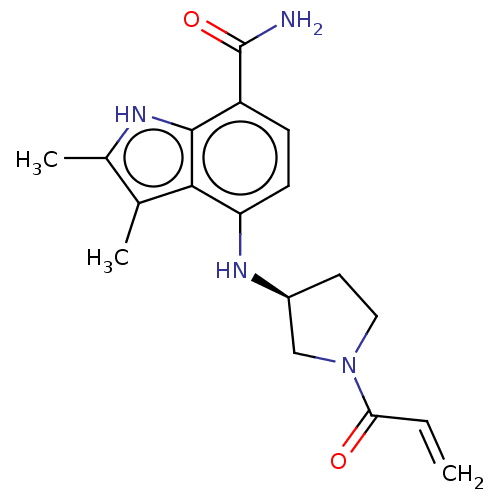 (CHEMBL4640555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50540958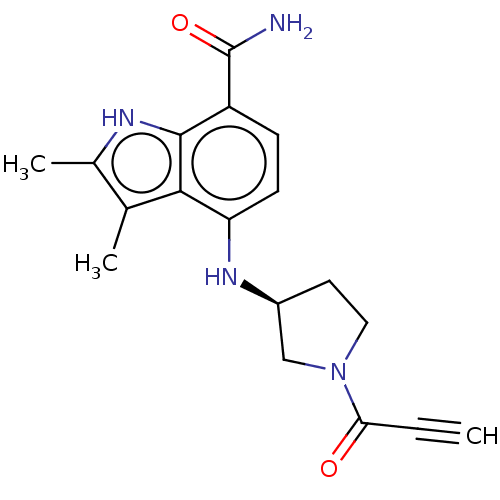 (CHEMBL4649284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165287 (US10604504, Example 83 | US11623921, Example 83 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165283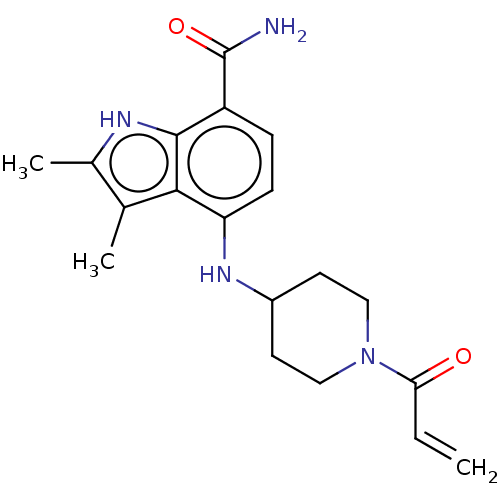 (US10604504, Example 93 | US11623921, Example 79 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165462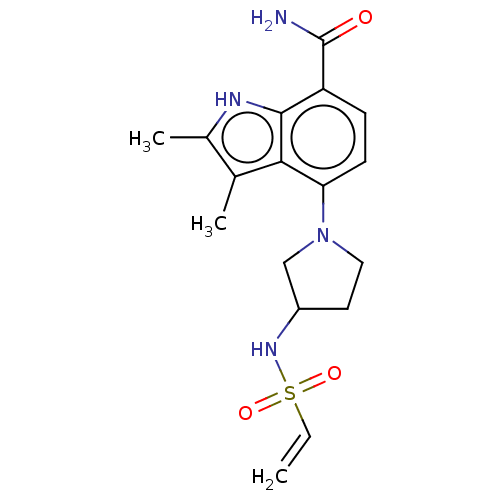 (US10604504, Example 140 | US11623921, Example 140 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165286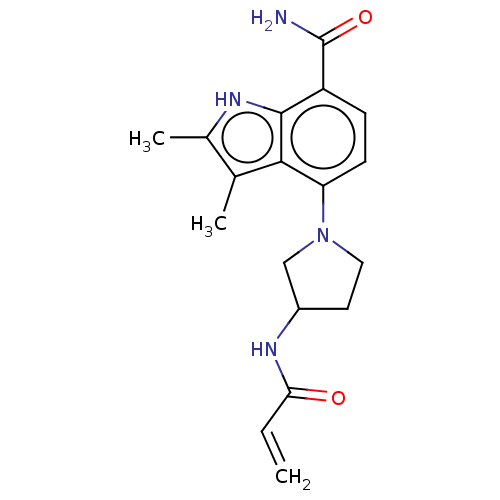 (US10604504, Example 82 | US11623921, Example 82 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of TXK (unknown origin) | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165323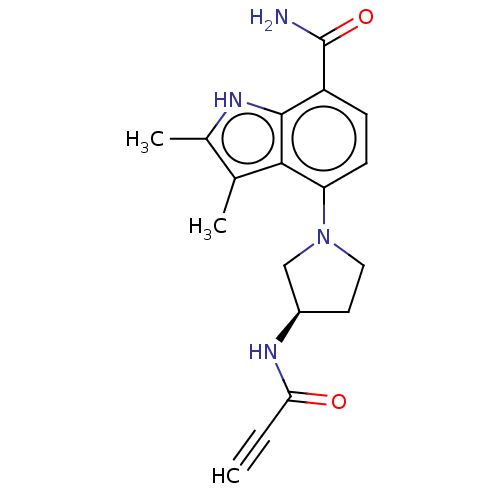 (US10604504, Example 119 | US11623921, Example 119 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50540957 (CHEMBL4640555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165323 (US10604504, Example 119 | US11623921, Example 119 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165291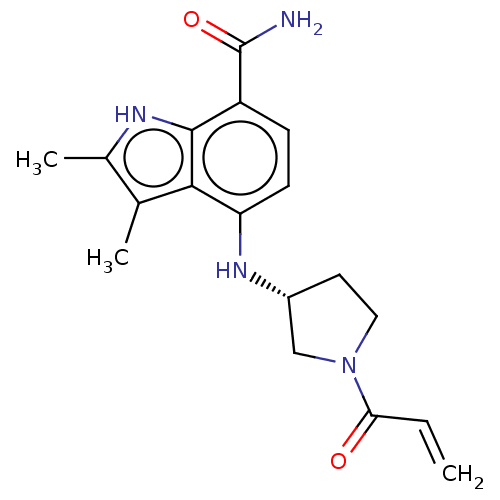 (US10604504, Example 87 | US11623921, Example 87 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50540958 (CHEMBL4649284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human peripheral B cells assessed as reduction in CD69 surface expression by whole blood assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165463 (US10604504, Example 141 | US11623921, Example 141 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165318 (US10604504, Example 114 | US11623921, Example 114 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165319 (US10604504, Example 115 | US11623921, Example 115 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165463 (US10604504, Example 141 | US11623921, Example 141 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human whole blood cells after 120 mins by ELISA | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR-2 | Drug Metab Dispos 39: 891-903 (2011) Article DOI: 10.1124/dmd.110.037341 BindingDB Entry DOI: 10.7270/Q2F191FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50088495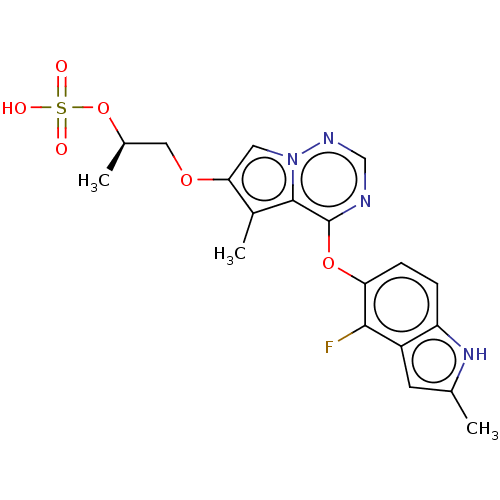 (CHEMBL3527569) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant FGFR-1 | Drug Metab Dispos 39: 891-903 (2011) Article DOI: 10.1124/dmd.110.037341 BindingDB Entry DOI: 10.7270/Q2F191FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165321 (US10604504, Example 117 | US11623921, Example 117 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50088495 (CHEMBL3527569) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR-2 | Drug Metab Dispos 39: 891-903 (2011) Article DOI: 10.1124/dmd.110.037341 BindingDB Entry DOI: 10.7270/Q2F191FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant FGFR-1 | Drug Metab Dispos 39: 891-903 (2011) Article DOI: 10.1124/dmd.110.037341 BindingDB Entry DOI: 10.7270/Q2F191FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165465 (US10604504, Example 143 | US11623921, Example 143 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165310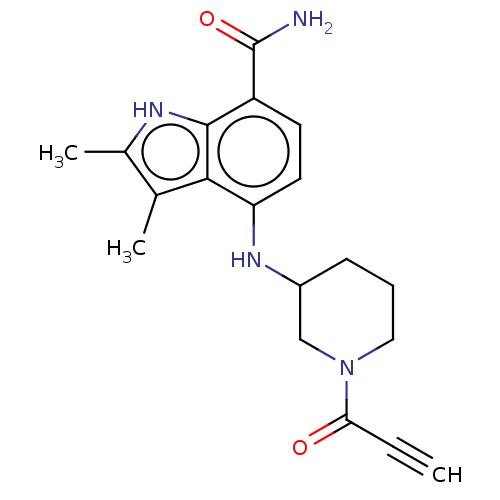 (US10604504, Example 106 | US11623921, Example 106 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HER4 (unknown origin) | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HER1 (unknown origin) | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165461 (US10604504, Example 139 | US11623921, Example 139 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165320 (US10604504, Example 116 | US11623921, Example 116 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165283 (US10604504, Example 93 | US11623921, Example 79 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos B cells assessed as reduction in BCR-stimulated calcium flux after 1 hr in dark condition measured for 180 sec | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165463 (US10604504, Example 141 | US11623921, Example 141 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human whole blood cells after 1 hr by ELISA | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |