

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496900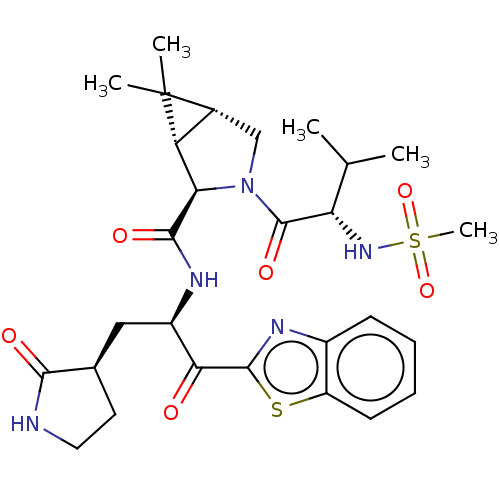 (science.abl4784, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496901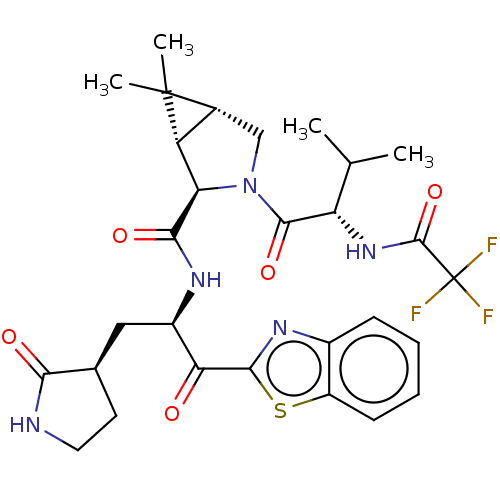 (science.abl4784, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496896 (US11312704, Compound 101 | US11351149, Example 49 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50301772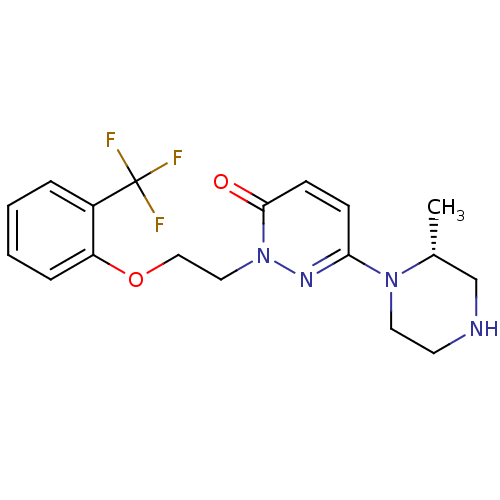 ((R)-6-(2-methylpiperazin-1-yl)-2-(2-(2-(trifluorom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA | Bioorg Med Chem Lett 19: 5791-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.136 BindingDB Entry DOI: 10.7270/Q2X0674Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496897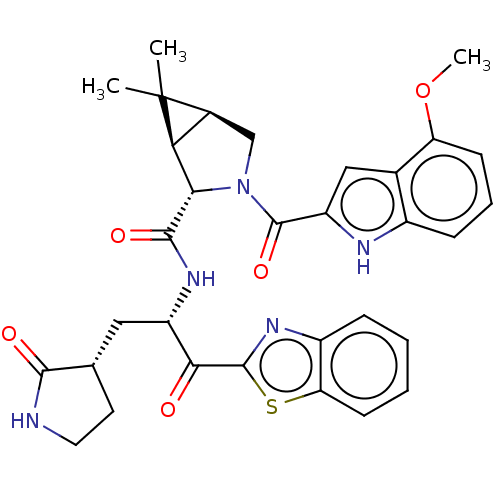 (science.abl4784, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50302145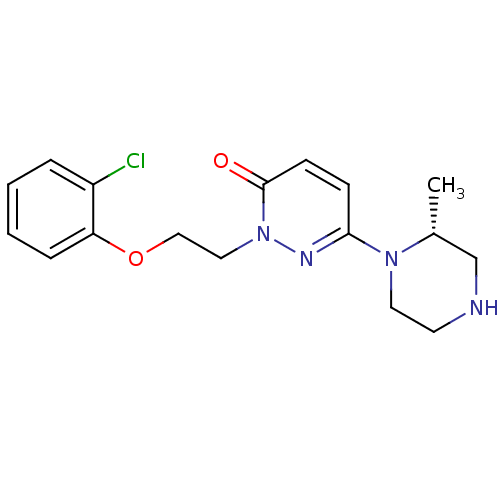 ((R)-2-(2-(2-chlorophenoxy)ethyl)-6-(2-methylpipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA | Bioorg Med Chem Lett 19: 5791-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.136 BindingDB Entry DOI: 10.7270/Q2X0674Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50302160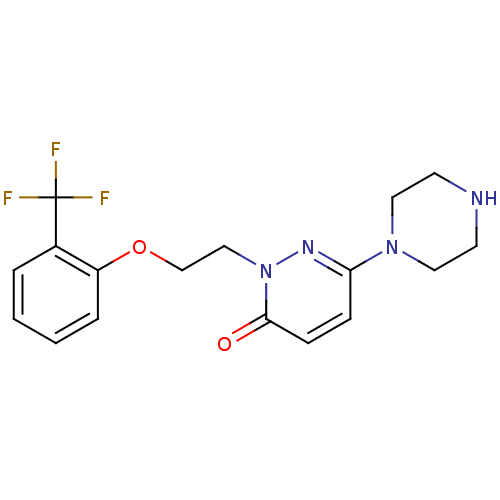 (6-(piperazin-1-yl)-2-(2-(2-(trifluoromethyl)phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA | Bioorg Med Chem Lett 19: 5791-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.136 BindingDB Entry DOI: 10.7270/Q2X0674Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50302151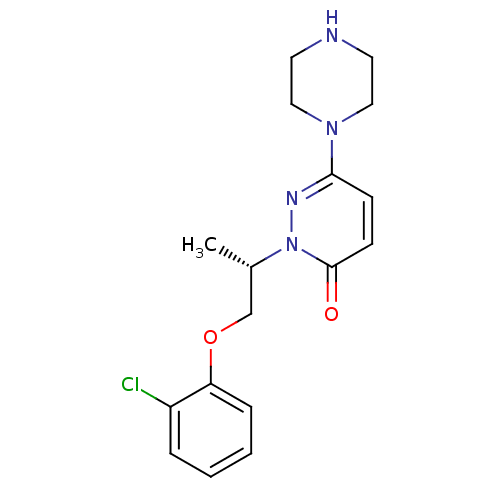 ((S)-2-(1-(2-chlorophenoxy)propan-2-yl)-6-(piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 728 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA | Bioorg Med Chem Lett 19: 5791-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.136 BindingDB Entry DOI: 10.7270/Q2X0674Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50226008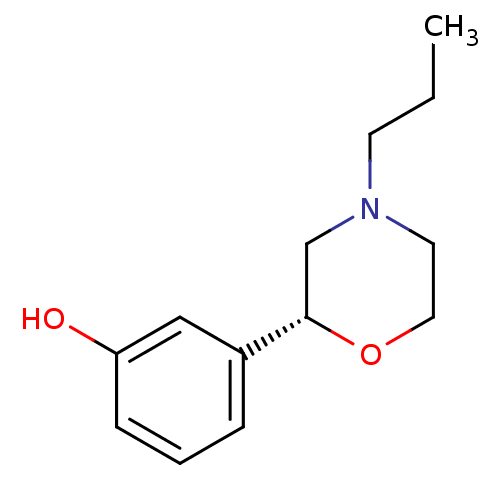 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to rat 5HT2A receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50226008 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to rat alpha1A adrenergic receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50226008 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human alpha2A adrenergic receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50226008 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M1 receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50302141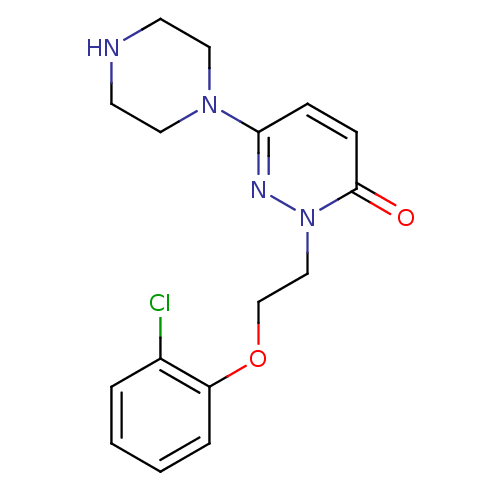 (2-(2-(2-chlorophenoxy)ethyl)-6-(piperazin-1-yl)pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA | Bioorg Med Chem Lett 19: 5791-5 (2009) Article DOI: 10.1016/j.bmcl.2009.07.136 BindingDB Entry DOI: 10.7270/Q2X0674Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50226008 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50226008 ((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of dofetilide from hERG | Bioorg Med Chem Lett 17: 6691-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.059 BindingDB Entry DOI: 10.7270/Q23J3CQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50301770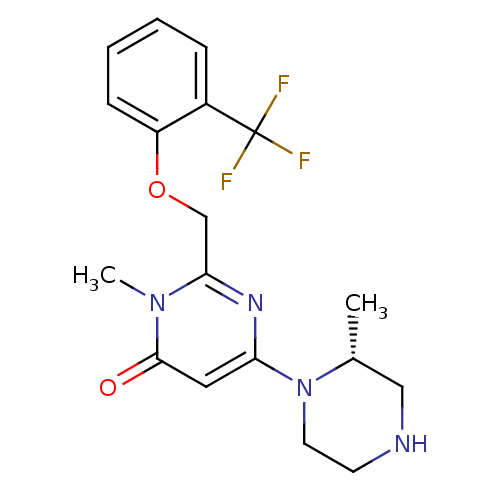 ((R)-3-methyl-6-(2-methylpiperazin-1-yl)-2-((2-(tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 19: 5346-50 (2009) Article DOI: 10.1016/j.bmcl.2009.07.133 BindingDB Entry DOI: 10.7270/Q2B56JT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126462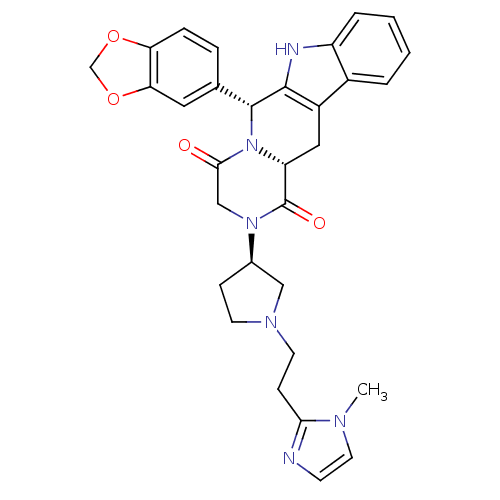 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit gamma (Canis lupus familiaris (Dog)) | BDBM14397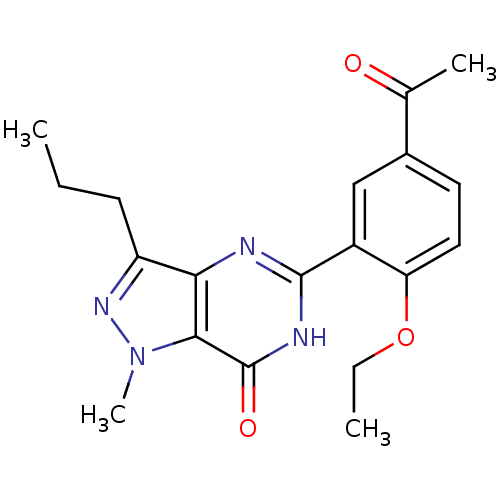 (5-(5-acetyl-2-ethoxyphenyl)-1-methyl-3-propyl-1H,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14408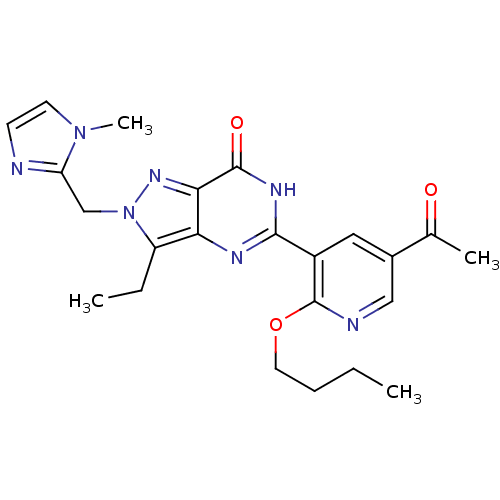 (5-(2-butoxy-5-acetylpyridin-3-yl)-3-ethyl-2-[(1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126467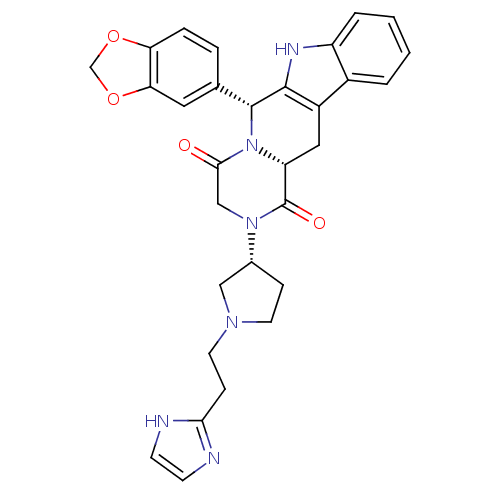 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14392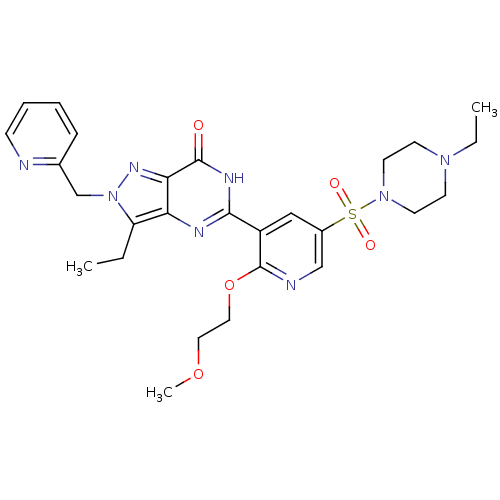 (3-ethyl-5-{5-[(4-ethylpiperazine-1-)sulfonyl]-2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit gamma (Canis lupus familiaris (Dog)) | BDBM14396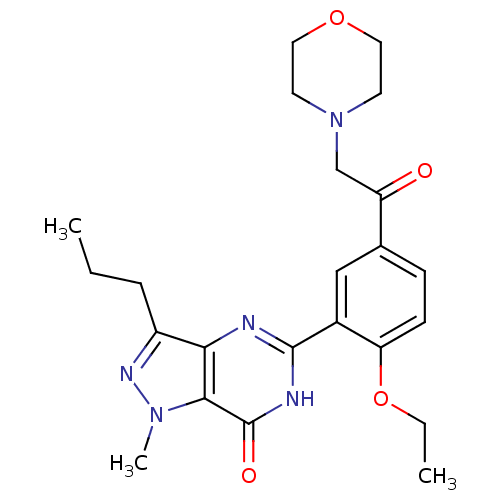 (5-{2-ethoxy-5-[2-(morpholin-4-yl)acetyl]phenyl}-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126462 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1-m...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126452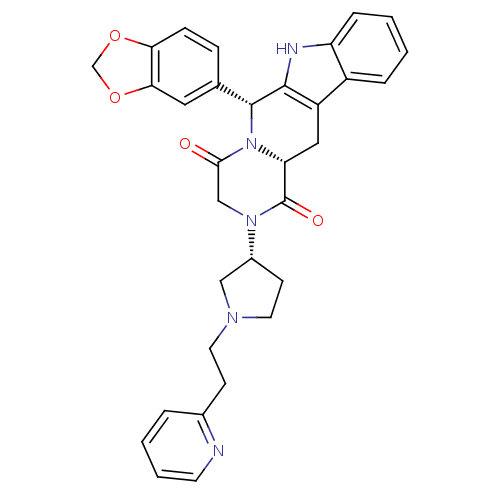 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14396 (5-{2-ethoxy-5-[2-(morpholin-4-yl)acetyl]phenyl}-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126455 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126464 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126452 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126458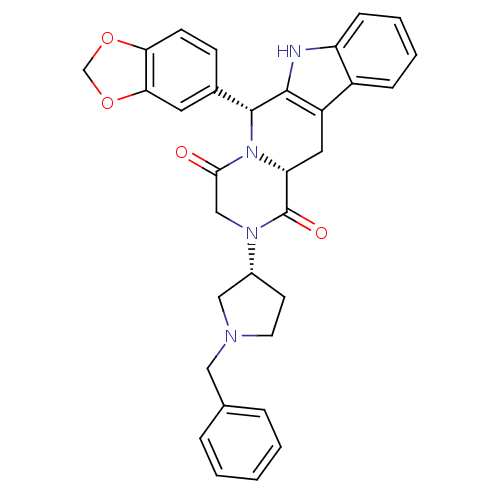 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-benzyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126455 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyri...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126449 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14407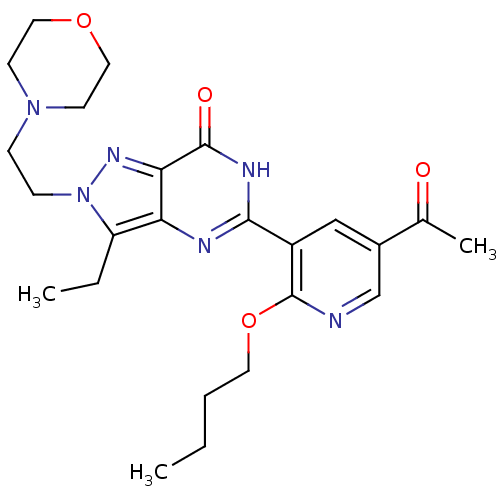 (5-(2-butoxy-5-acetylpyridin-3-yl)-3-ethyl-2-[2-(mo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126451 ((6R,12aR)-2-((R)-1-Benzyl-pyrrolidin-3-yl)-6-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14398 (5-(5-Acetyl-2-propoxy-3-pyridinyl)-3-ethyl-2-[(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14394 (5-(2-butoxy-5-acetylpyridin-3-yl)-3-ethyl-2-(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126467 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-{(R)-1-[2-(1H-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126453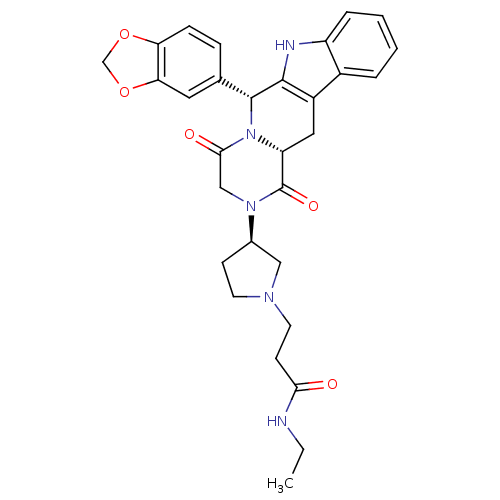 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14413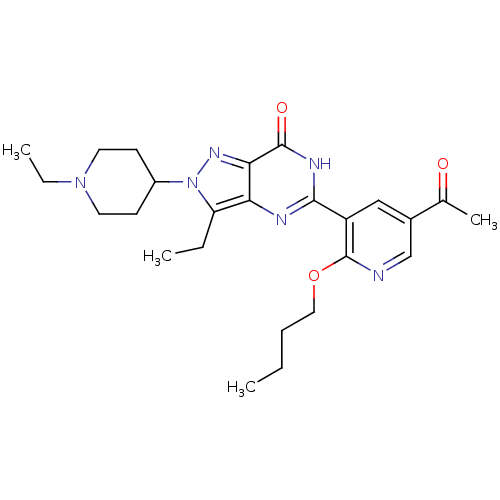 (5-(2-butoxy-5-acetylpyridin-3-yl)-3-ethyl-2-(1-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126448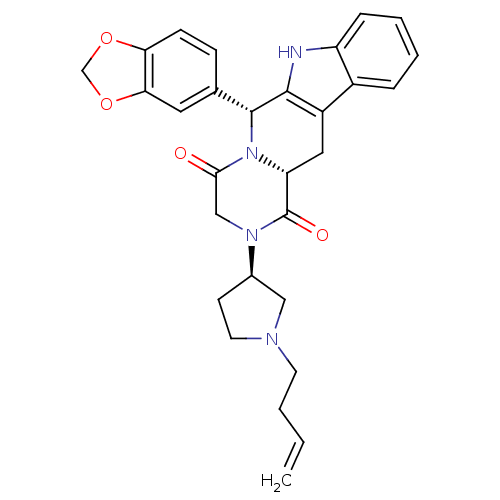 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-but-3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50126456 ((6R,12aR)-2-[(R)-1-(3-Azetidin-1-yl-3-oxo-propyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 5 (PDE5) obtained from human corpus cavernosum tissue | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126464 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-[(R)-1-(2-pyra...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126453 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126449 (3-[(R)-3-((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-1,4-di...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (Homo sapiens (Human)) | BDBM50126456 ((6R,12aR)-2-[(R)-1-(3-Azetidin-1-yl-3-oxo-propyl)-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 11 (PDE11) obtained from recombinant Sf9 expression | Bioorg Med Chem Lett 13: 1425-8 (2003) BindingDB Entry DOI: 10.7270/Q2GM86N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14402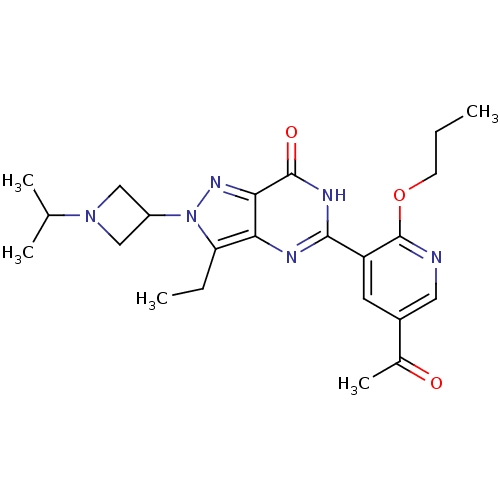 (5-(5-Acetyl-2-propoxy-3-pyridinyl)-3-ethyl-2-(1-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE5 or PDE6 catalytic activity was monitored by measuring the hydrolysis of [3H]-cGMP to [3H]-GMP using a scintillation proximity assay (SPA). [3H]-... | J Med Chem 49: 3581-94 (2006) Article DOI: 10.1021/jm060113e BindingDB Entry DOI: 10.7270/Q2C24TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 282 total ) | Next | Last >> |