

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702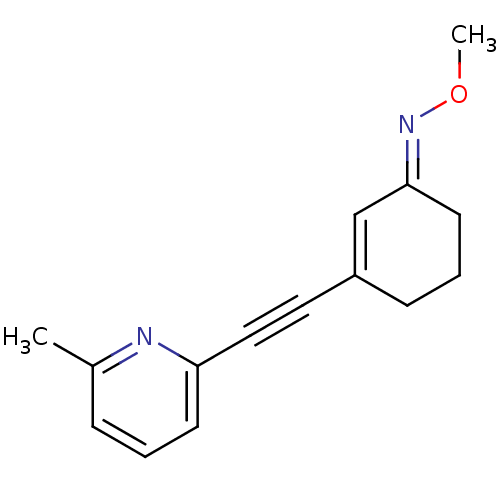 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50123005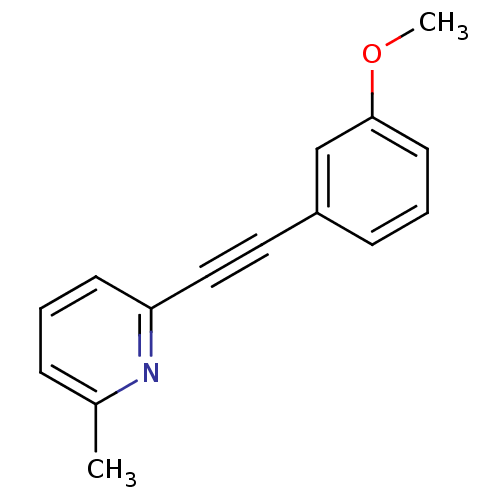 (2-(3-Methoxy-phenylethynyl)-6-methyl-pyridine | 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50123005 (2-(3-Methoxy-phenylethynyl)-6-methyl-pyridine | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137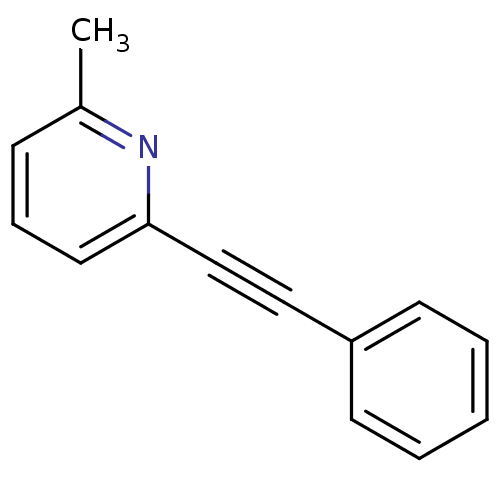 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50084137 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50094186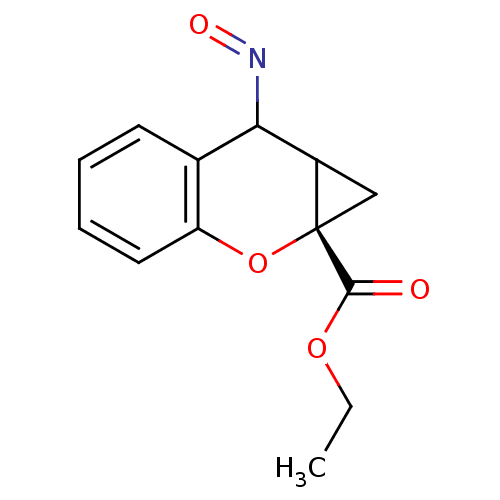 ((-)1aS,7aS -2-Hydroxyimino-1a,2-dihydro-1H-7-oxa-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50094186 ((-)1aS,7aS -2-Hydroxyimino-1a,2-dihydro-1H-7-oxa-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120943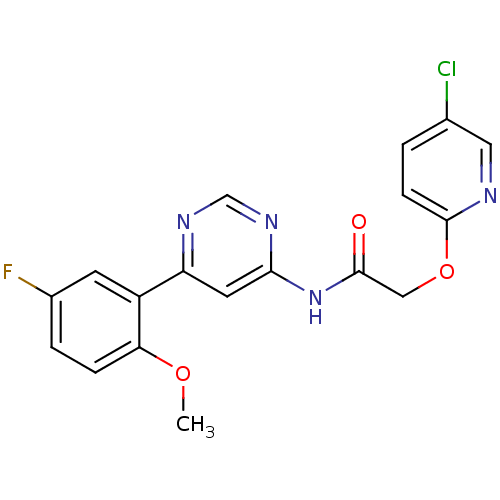 (US8716296, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120941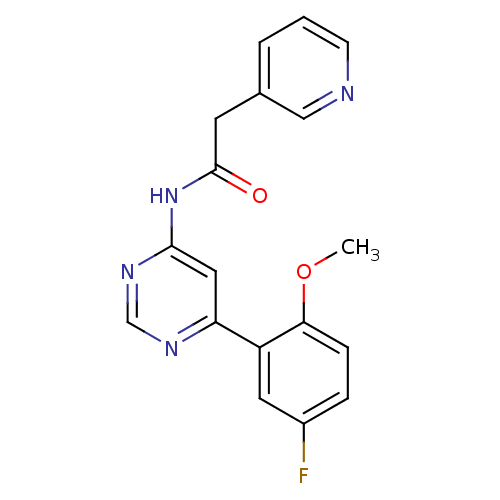 (US8716296, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120934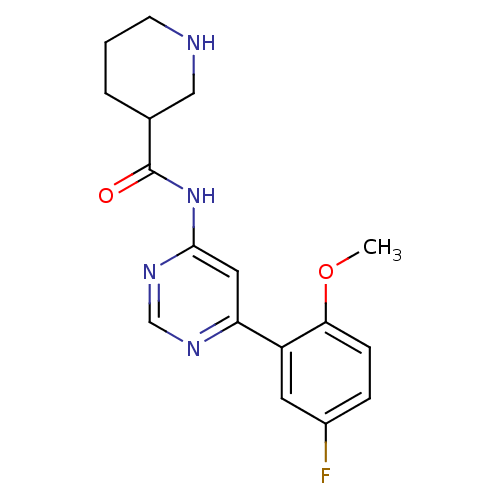 (US8716296, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120936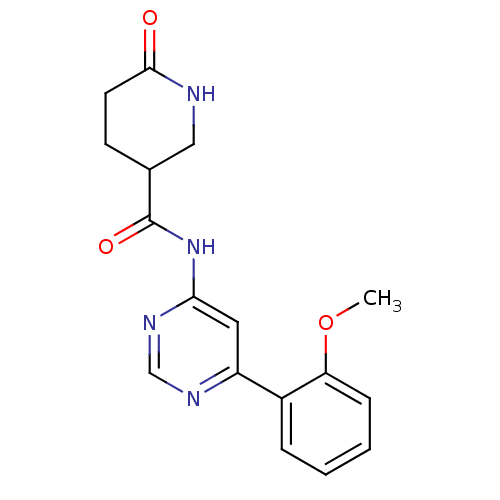 (US8716296, 5 | US8716296, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120942 (US8716296, 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120935 (US8716296, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120939 (US8716296, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.116 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120936 (US8716296, 5 | US8716296, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.173 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50451503 (CHEMBL12513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451505 (CHEMBL274422) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120940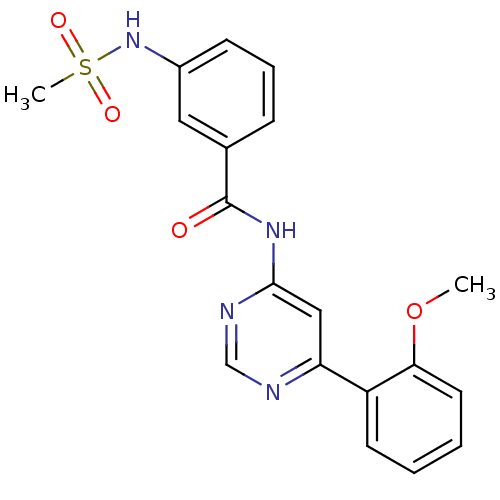 (US8716296, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50451505 (CHEMBL274422) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120938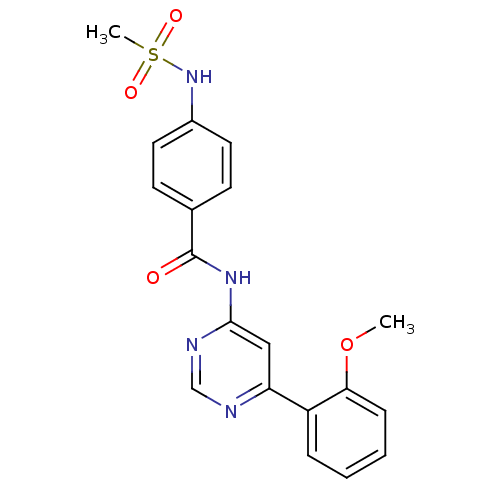 (US8716296, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451503 (CHEMBL12513) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2A heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of glutamate-induced calcium release | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of quisqualate-induced phosphoinositol accumulation | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50451498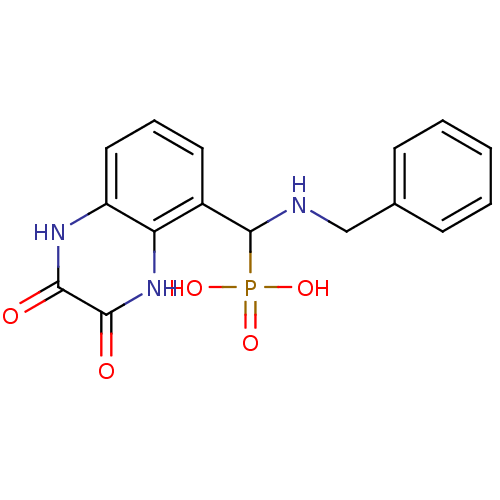 (CHEMBL536107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069471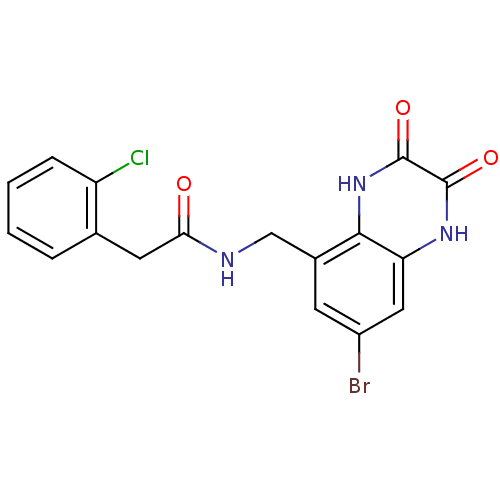 (CHEMBL357190 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM120933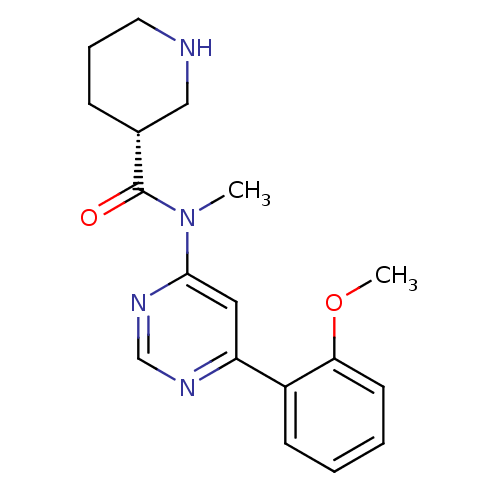 (US8716296, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Ingenium Pharmaceuticals GmbH US Patent | Assay Description All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt... | US Patent US8716296 (2014) BindingDB Entry DOI: 10.7270/Q2PN949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069483 (CHEMBL356281 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069482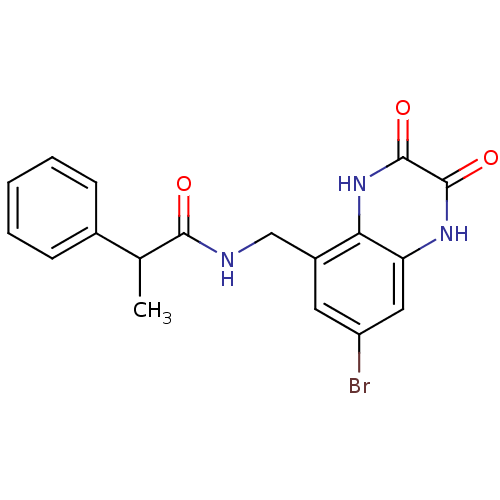 (CHEMBL357916 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069465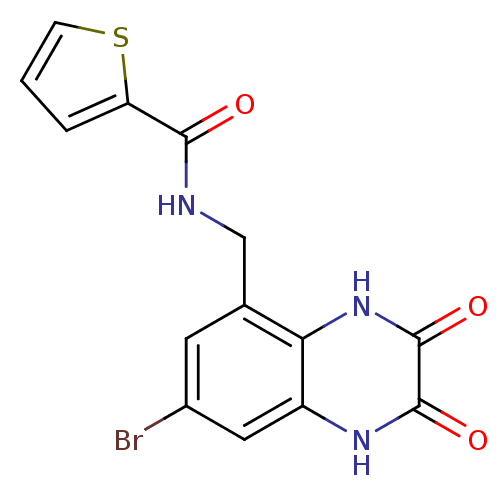 (CHEMBL343863 | Thiophene-2-carboxylic acid (7-brom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069459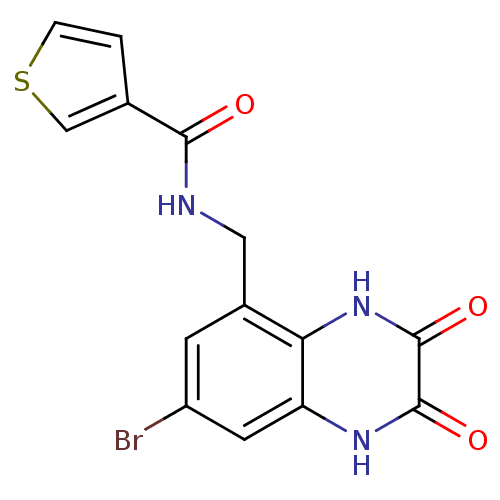 (CHEMBL413125 | Thiophene-3-carboxylic acid (7-brom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069458 (CHEMBL147895 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069495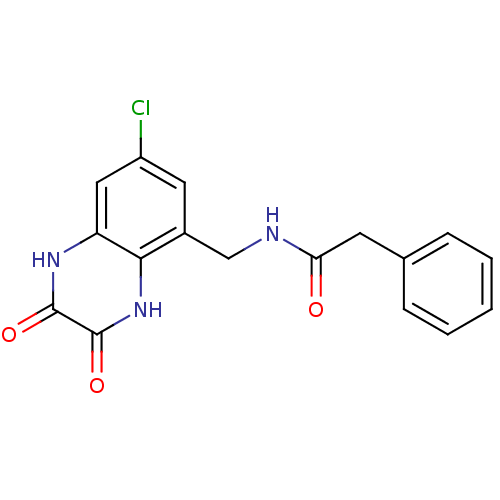 (CHEMBL147894 | N-(7-Chloro-2,3-dioxo-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50451509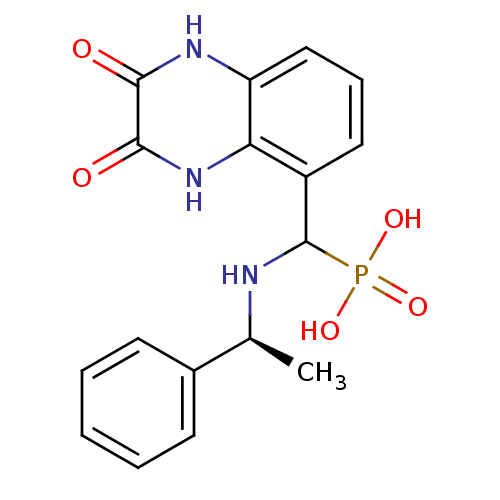 (CHEMBL268920) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069478 (3-Methyl-thiophene-2-carboxylic acid (7-bromo-2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069456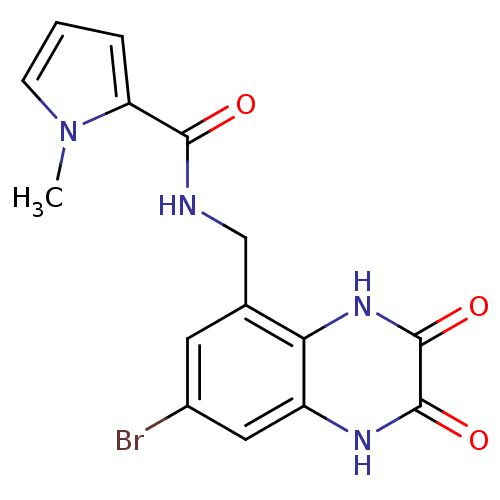 (1-Methyl-1H-pyrrole-2-carboxylic acid (7-bromo-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069461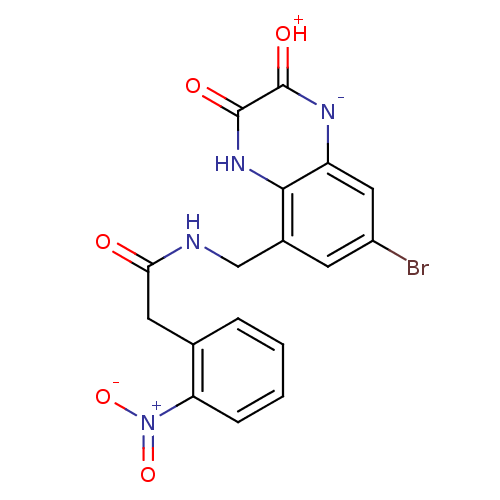 (CHEMBL357447 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069470 (CHEMBL145606 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069491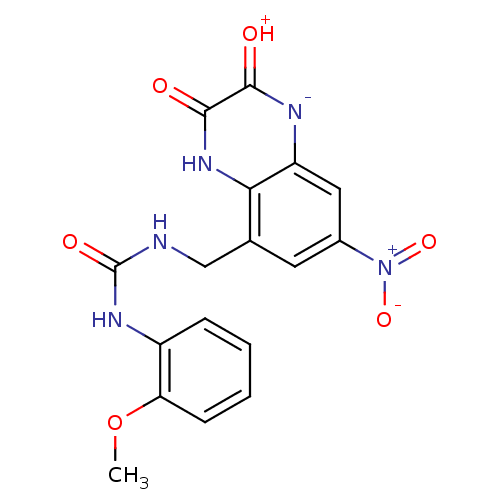 (1-(2-Methoxy-phenyl)-3-(7-nitro-2,3-dioxo-1,2,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069479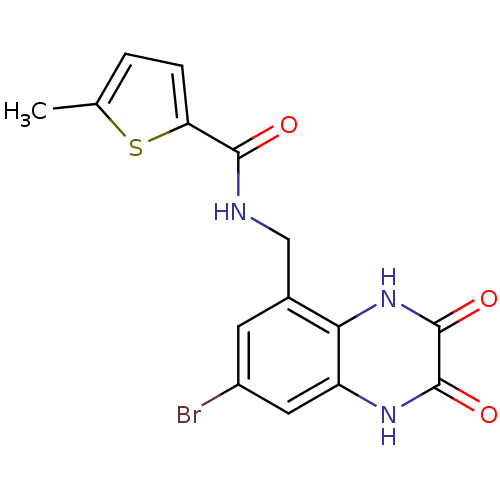 (5-Methyl-thiophene-2-carboxylic acid (7-bromo-2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069488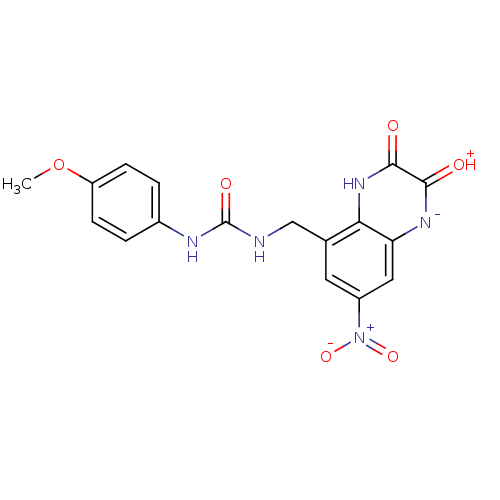 (1-(4-Methoxy-phenyl)-3-(7-nitro-2,3-dioxo-1,2,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50451498 (CHEMBL536107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069464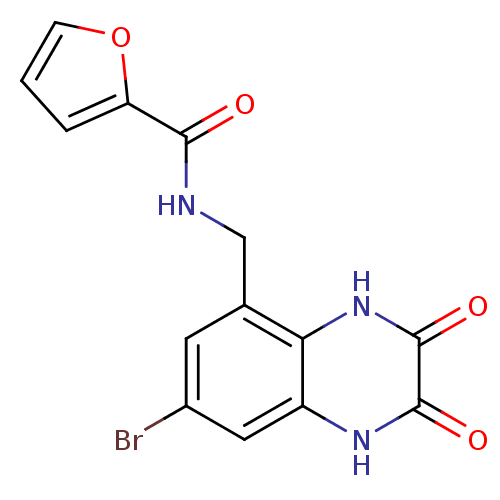 (CHEMBL144064 | Furan-2-carboxylic acid (7-bromo-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069460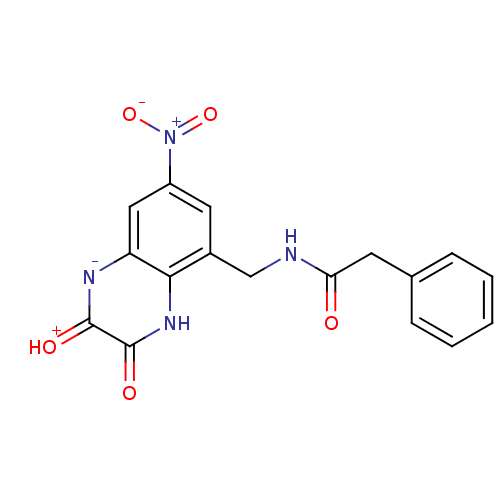 (CHEMBL144365 | N-(7-Nitro-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50451518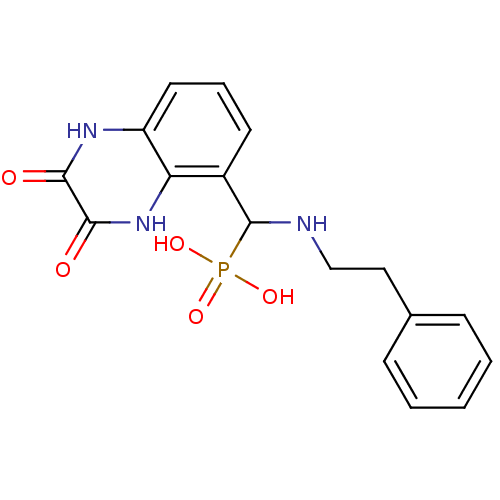 (CHEMBL12256) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptor | Bioorg Med Chem Lett 12: 1099-102 (2002) BindingDB Entry DOI: 10.7270/Q24B31V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069467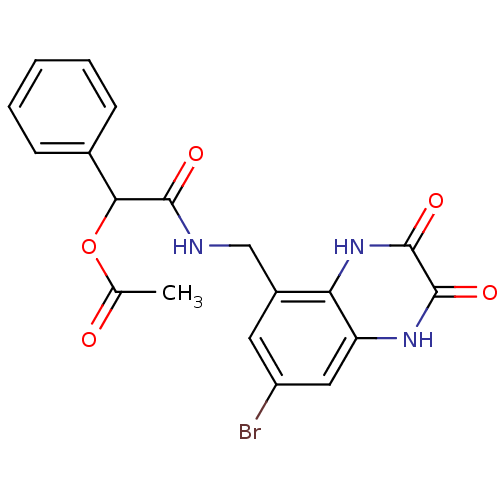 (Acetic acid [(7-bromo-2,3-dioxo-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069497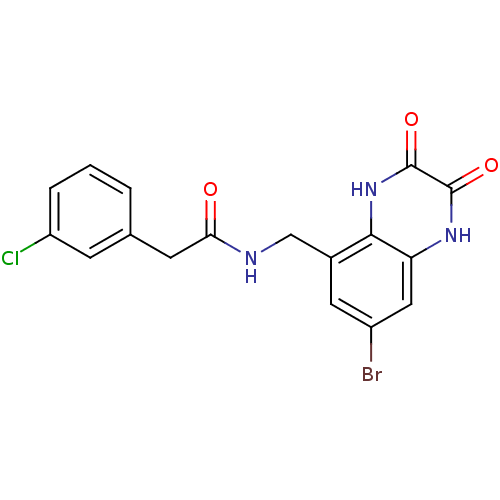 (CHEMBL146827 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50069490 (CHEMBL147855 | N-(7-Bromo-2,3-dioxo-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors | Bioorg Med Chem Lett 8: 493-8 (1999) BindingDB Entry DOI: 10.7270/Q29G5NBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |