 Found 39 hits with Last Name = 'alm' and Initial = 'ra'
Found 39 hits with Last Name = 'alm' and Initial = 'ra' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate racemase
(Helicobacter pylori) | BDBM26431
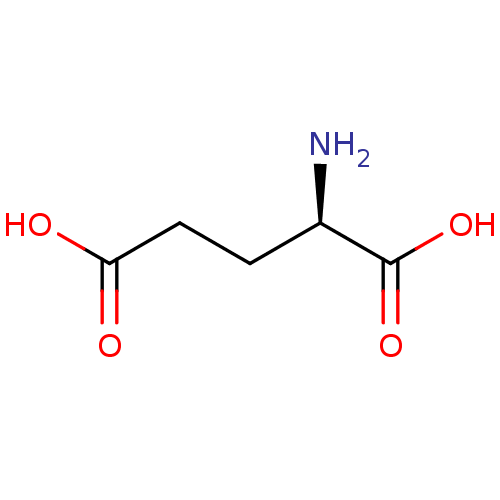
((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate racemase
(Helicobacter pylori) | BDBM50215445
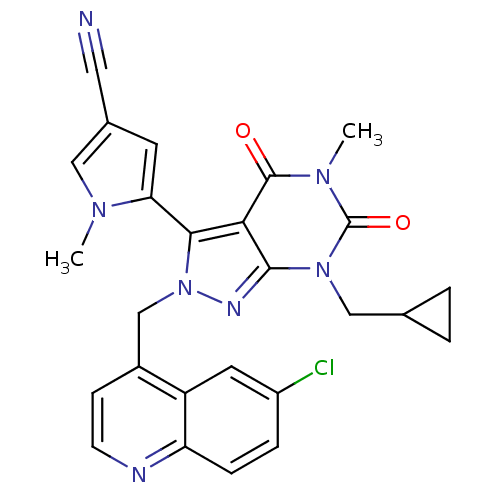
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444
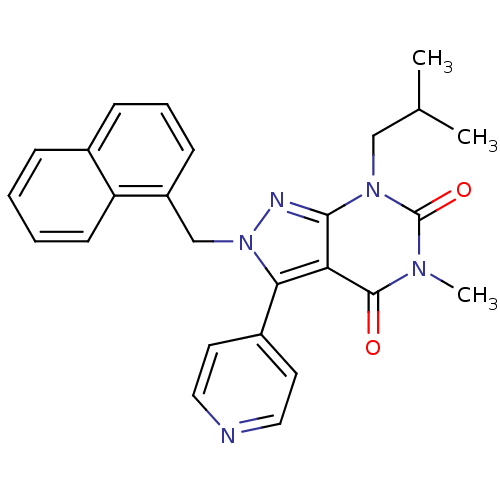
(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori glutamate racemase |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395412
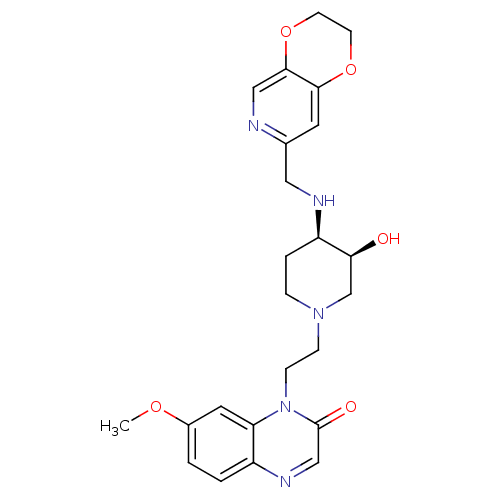
(CHEMBL2164745)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@@H](NCc4cc5OCCOc5cn4)[C@@H](O)C3)c2c1 |r| Show InChI InChI=1S/C24H29N5O5/c1-32-17-2-3-18-20(11-17)29(24(31)14-27-18)7-6-28-5-4-19(21(30)15-28)26-12-16-10-22-23(13-25-16)34-9-8-33-22/h2-3,10-11,13-14,19,21,26,30H,4-9,12,15H2,1H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395418

(CHEMBL2164739)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@H](NCc4ccc5OCC(=O)Nc5n4)[C@@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-34-16-3-4-19-20(10-16)31(23(33)12-27-19)9-8-30-7-6-18(17(25)13-30)26-11-15-2-5-21-24(28-15)29-22(32)14-35-21/h2-5,10,12,17-18,26H,6-9,11,13-14H2,1H3,(H,28,29,32)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395395
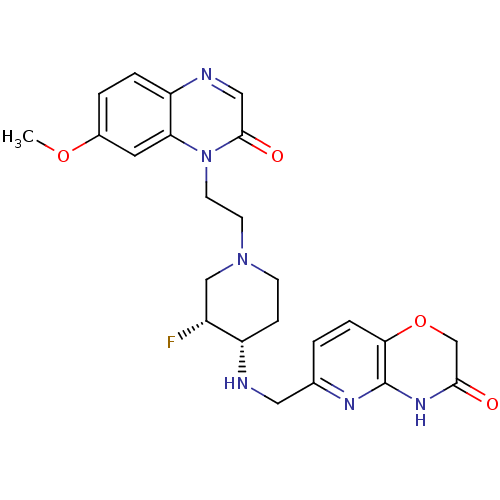
(CHEMBL2163364)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@H](NCc4ccc5OCC(=O)Nc5n4)[C@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-34-16-3-4-19-20(10-16)31(23(33)12-27-19)9-8-30-7-6-18(17(25)13-30)26-11-15-2-5-21-24(28-15)29-22(32)14-35-21/h2-5,10,12,17-18,26H,6-9,11,13-14H2,1H3,(H,28,29,32)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395401
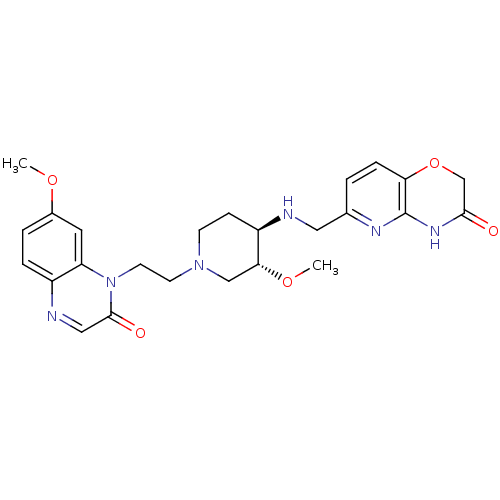
(CHEMBL2165061)Show SMILES CO[C@@H]1CN(CCn2c3cc(OC)ccc3ncc2=O)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H30N6O5/c1-34-17-4-5-18-20(11-17)31(24(33)13-27-18)10-9-30-8-7-19(22(14-30)35-2)26-12-16-3-6-21-25(28-16)29-23(32)15-36-21/h3-6,11,13,19,22,26H,7-10,12,14-15H2,1-2H3,(H,28,29,32)/t19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395394
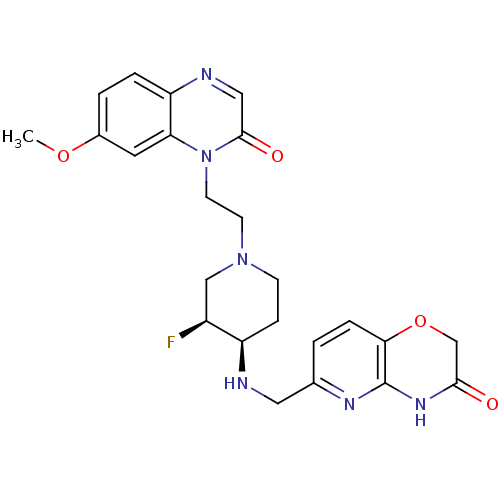
(CHEMBL2165067)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@@H](NCc4ccc5OCC(=O)Nc5n4)[C@@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H27FN6O4/c1-34-16-3-4-19-20(10-16)31(23(33)12-27-19)9-8-30-7-6-18(17(25)13-30)26-11-15-2-5-21-24(28-15)29-22(32)14-35-21/h2-5,10,12,17-18,26H,6-9,11,13-14H2,1H3,(H,28,29,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395413
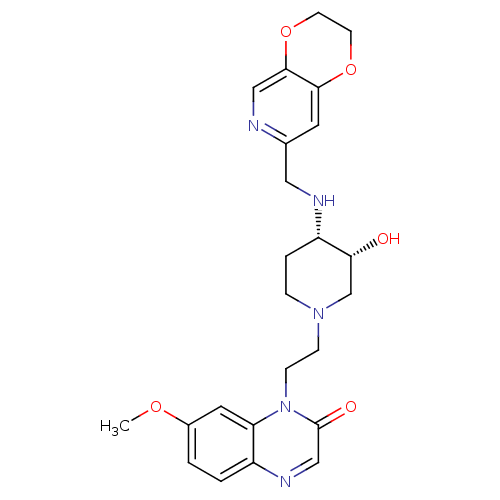
(CHEMBL2164744)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@H](NCc4cc5OCCOc5cn4)[C@H](O)C3)c2c1 |r| Show InChI InChI=1S/C24H29N5O5/c1-32-17-2-3-18-20(11-17)29(24(31)14-27-18)7-6-28-5-4-19(21(30)15-28)26-12-16-10-22-23(13-25-16)34-9-8-33-22/h2-3,10-11,13-14,19,21,26,30H,4-9,12,15H2,1H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395399
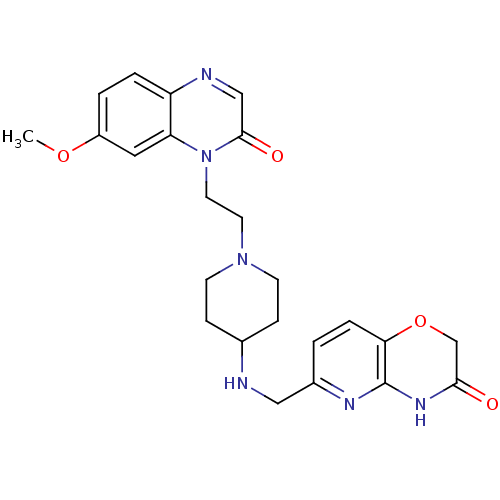
(CHEMBL2165063)Show SMILES COc1ccc2ncc(=O)n(CCN3CCC(CC3)NCc3ccc4OCC(=O)Nc4n3)c2c1 Show InChI InChI=1S/C24H28N6O4/c1-33-18-3-4-19-20(12-18)30(23(32)14-26-19)11-10-29-8-6-16(7-9-29)25-13-17-2-5-21-24(27-17)28-22(31)15-34-21/h2-5,12,14,16,25H,6-11,13,15H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395400
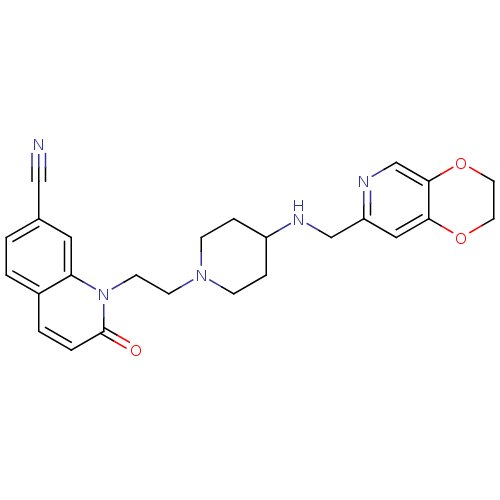
(CHEMBL2165062)Show SMILES O=c1ccc2ccc(cc2n1CCN1CCC(CC1)NCc1cc2OCCOc2cn1)C#N Show InChI InChI=1S/C25H27N5O3/c26-15-18-1-2-19-3-4-25(31)30(22(19)13-18)10-9-29-7-5-20(6-8-29)27-16-21-14-23-24(17-28-21)33-12-11-32-23/h1-4,13-14,17,20,27H,5-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395400

(CHEMBL2165062)Show SMILES O=c1ccc2ccc(cc2n1CCN1CCC(CC1)NCc1cc2OCCOc2cn1)C#N Show InChI InChI=1S/C25H27N5O3/c26-15-18-1-2-19-3-4-25(31)30(22(19)13-18)10-9-29-7-5-20(6-8-29)27-16-21-14-23-24(17-28-21)33-12-11-32-23/h1-4,13-14,17,20,27H,5-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395393

(CHEMBL2165068)Show SMILES F[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395419
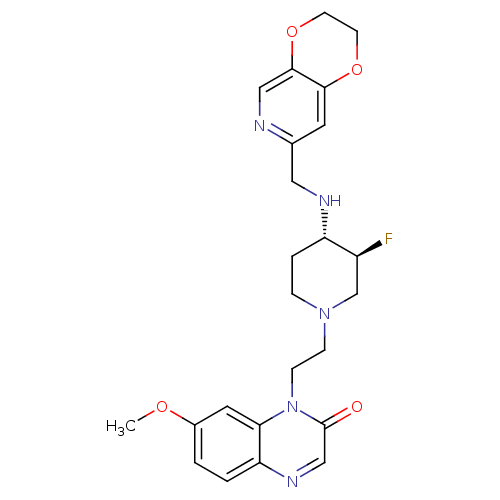
(CHEMBL2165069)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@H](NCc4cc5OCCOc5cn4)[C@@H](F)C3)c2c1 |r| Show InChI InChI=1S/C24H28FN5O4/c1-32-17-2-3-20-21(11-17)30(24(31)14-28-20)7-6-29-5-4-19(18(25)15-29)27-12-16-10-22-23(13-26-16)34-9-8-33-22/h2-3,10-11,13-14,18-19,27H,4-9,12,15H2,1H3/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395406
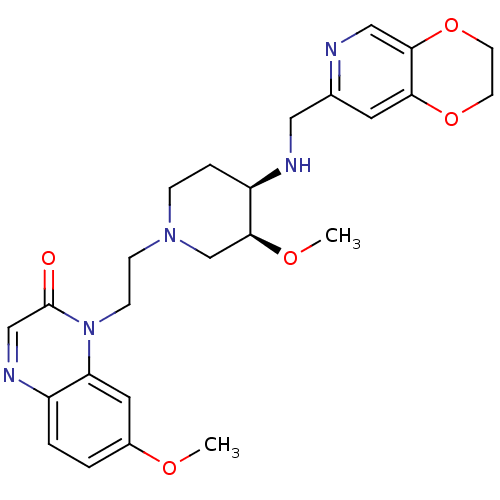
(CHEMBL2165056)Show SMILES CO[C@H]1CN(CCn2c3cc(OC)ccc3ncc2=O)CC[C@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H31N5O5/c1-32-18-3-4-19-21(12-18)30(25(31)15-28-19)8-7-29-6-5-20(24(16-29)33-2)27-13-17-11-22-23(14-26-17)35-10-9-34-22/h3-4,11-12,14-15,20,24,27H,5-10,13,16H2,1-2H3/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395405

(CHEMBL2165057)Show SMILES CO[C@H]1CN(CCn2c3cc(OC)ccc3ncc2=O)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H30N6O5/c1-34-17-4-5-18-20(11-17)31(24(33)13-27-18)10-9-30-8-7-19(22(14-30)35-2)26-12-16-3-6-21-25(28-16)29-23(32)15-36-21/h3-6,11,13,19,22,26H,7-10,12,14-15H2,1-2H3,(H,28,29,32)/t19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395402
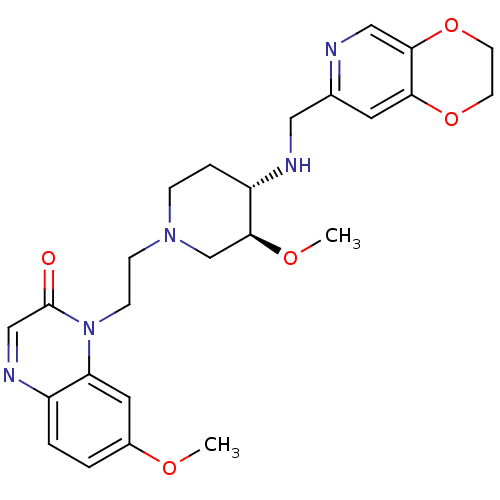
(CHEMBL2165060)Show SMILES CO[C@H]1CN(CCn2c3cc(OC)ccc3ncc2=O)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H31N5O5/c1-32-18-3-4-19-21(12-18)30(25(31)15-28-19)8-7-29-6-5-20(24(16-29)33-2)27-13-17-11-22-23(14-26-17)35-10-9-34-22/h3-4,11-12,14-15,20,24,27H,5-10,13,16H2,1-2H3/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395409

(CHEMBL2164748)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@@H](NCc4cc5OCCOc5cn4)[C@H](O)C3)c2c1 |r| Show InChI InChI=1S/C24H29N5O5/c1-32-17-2-3-18-20(11-17)29(24(31)14-27-18)7-6-28-5-4-19(21(30)15-28)26-12-16-10-22-23(13-25-16)34-9-8-33-22/h2-3,10-11,13-14,19,21,26,30H,4-9,12,15H2,1H3/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395416

(CHEMBL2164741)Show SMILES O[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H27N5O4/c26-13-17-1-2-18-3-4-25(32)30(21(18)11-17)8-7-29-6-5-20(22(31)16-29)28-14-19-12-23-24(15-27-19)34-10-9-33-23/h1-4,11-12,15,20,22,28,31H,5-10,14,16H2/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395393

(CHEMBL2165068)Show SMILES F[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395403

(CHEMBL2165059)Show SMILES CO[C@@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C26H28N6O4/c1-35-23-15-31(10-11-32-21-12-17(13-27)2-3-18(21)4-7-25(32)34)9-8-20(23)28-14-19-5-6-22-26(29-19)30-24(33)16-36-22/h2-7,12,20,23,28H,8-11,14-16H2,1H3,(H,29,30,33)/t20-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395410

(CHEMBL2164747)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@@H](NCc4ccc5OCC(=O)Nc5n4)[C@@H](O)C3)c2c1 |r| Show InChI InChI=1S/C24H28N6O5/c1-34-16-3-4-17-19(10-16)30(23(33)12-26-17)9-8-29-7-6-18(20(31)13-29)25-11-15-2-5-21-24(27-15)28-22(32)14-35-21/h2-5,10,12,18,20,25,31H,6-9,11,13-14H2,1H3,(H,27,28,32)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395417
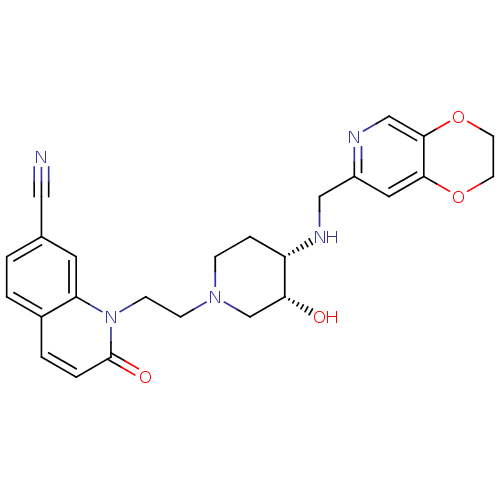
(CHEMBL2164740)Show SMILES O[C@@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H27N5O4/c26-13-17-1-2-18-3-4-25(32)30(21(18)11-17)8-7-29-6-5-20(22(31)16-29)28-14-19-12-23-24(15-27-19)34-10-9-33-23/h1-4,11-12,15,20,22,28,31H,5-10,14,16H2/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395396
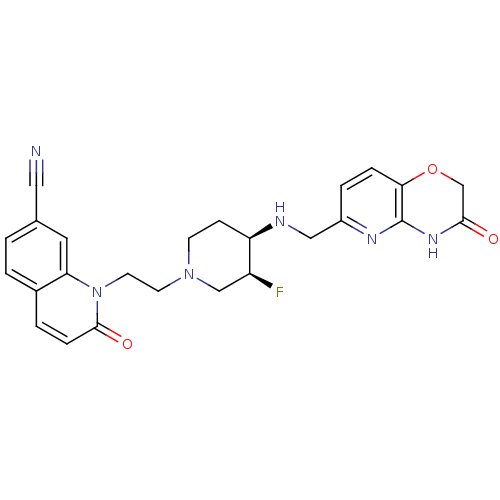
(CHEMBL2165066)Show SMILES F[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H25FN6O3/c26-19-14-31(9-10-32-21-11-16(12-27)1-2-17(21)3-6-24(32)34)8-7-20(19)28-13-18-4-5-22-25(29-18)30-23(33)15-35-22/h1-6,11,19-20,28H,7-10,13-15H2,(H,29,30,33)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395411
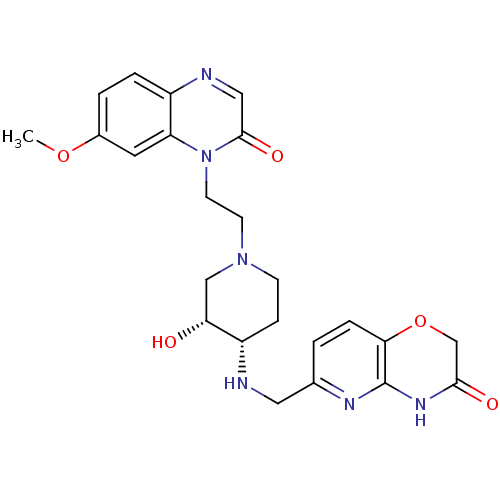
(CHEMBL2164746)Show SMILES COc1ccc2ncc(=O)n(CCN3CC[C@H](NCc4ccc5OCC(=O)Nc5n4)[C@H](O)C3)c2c1 |r| Show InChI InChI=1S/C24H28N6O5/c1-34-16-3-4-17-19(10-16)30(23(33)12-26-17)9-8-29-7-6-18(20(31)13-29)25-11-15-2-5-21-24(27-15)28-22(32)14-35-21/h2-5,10,12,18,20,25,31H,6-9,11,13-14H2,1H3,(H,27,28,32)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395408

(CHEMBL2165054)Show SMILES CO[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C26H29N5O4/c1-33-25-17-30(8-9-31-22-12-18(14-27)2-3-19(22)4-5-26(31)32)7-6-21(25)29-15-20-13-23-24(16-28-20)35-11-10-34-23/h2-5,12-13,16,21,25,29H,6-11,15,17H2,1H3/t21-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395404

(CHEMBL2165058)Show SMILES CO[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C26H29N5O4/c1-33-25-17-30(8-9-31-22-12-18(14-27)2-3-19(22)4-5-26(31)32)7-6-21(25)29-15-20-13-23-24(16-28-20)35-11-10-34-23/h2-5,12-13,16,21,25,29H,6-11,15,17H2,1H3/t21-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395407

(CHEMBL2165055)Show SMILES CO[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C26H28N6O4/c1-35-23-15-31(10-11-32-21-12-17(13-27)2-3-18(21)4-7-25(32)34)9-8-20(23)28-14-19-5-6-22-26(29-19)30-24(33)16-36-22/h2-7,12,20,23,28H,8-11,14-16H2,1H3,(H,29,30,33)/t20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395397

(CHEMBL2165065)Show SMILES F[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395397

(CHEMBL2165065)Show SMILES F[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395398
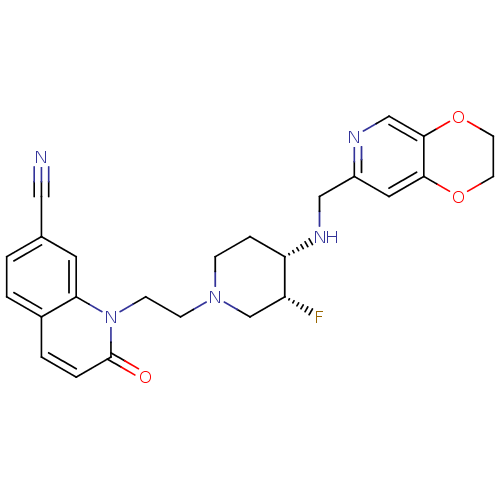
(CHEMBL2165064)Show SMILES F[C@@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-astemizole from human ERG expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395398

(CHEMBL2165064)Show SMILES F[C@@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1cc2OCCOc2cn1 |r| Show InChI InChI=1S/C25H26FN5O3/c26-20-16-30(7-8-31-22-11-17(13-27)1-2-18(22)3-4-25(31)32)6-5-21(20)29-14-19-12-23-24(15-28-19)34-10-9-33-23/h1-4,11-12,15,20-21,29H,5-10,14,16H2/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395415
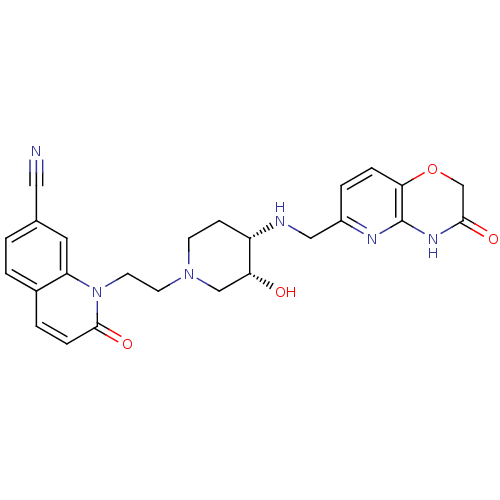
(CHEMBL2164742)Show SMILES O[C@@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H26N6O4/c26-12-16-1-2-17-3-6-24(34)31(20(17)11-16)10-9-30-8-7-19(21(32)14-30)27-13-18-4-5-22-25(28-18)29-23(33)15-35-22/h1-6,11,19,21,27,32H,7-10,13-15H2,(H,28,29,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50395414
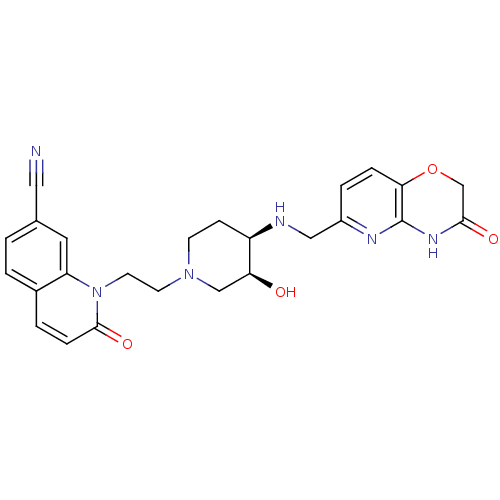
(CHEMBL2164743)Show SMILES O[C@H]1CN(CCn2c3cc(ccc3ccc2=O)C#N)CC[C@H]1NCc1ccc2OCC(=O)Nc2n1 |r| Show InChI InChI=1S/C25H26N6O4/c26-12-16-1-2-17-3-6-24(34)31(20(17)11-16)10-9-30-8-7-19(21(32)14-30)27-13-18-4-5-22-25(28-18)29-23(33)15-35-22/h1-6,11,19,21,27,32H,7-10,13-15H2,(H,28,29,33)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by Ionworks assay |
J Med Chem 55: 6916-33 (2012)
Article DOI: 10.1021/jm300690s
BindingDB Entry DOI: 10.7270/Q23B6188 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Streptococcus pneumoniae) | BDBM50215445

(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of MurI in Streptococcus pneumoniae |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Streptococcus pneumoniae) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of MurI in Streptococcus pneumoniae |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by isothermal titration calorimetry |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate racemase
(Helicobacter pylori) | BDBM50215445

(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by protein fluorescence binding assay |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215444

(5-METHYL-7-(2-METHYLPROPYL)-2-(NAPHTHALEN-1-YLMETH...)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3ccncc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C26H25N5O2/c1-17(2)15-30-24-22(25(32)29(3)26(30)33)23(19-11-13-27-14-12-19)31(28-24)16-20-9-6-8-18-7-4-5-10-21(18)20/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Helicobacter pylori MurI by protein fluorescence binding assay |
Nature 447: 817-822 (2007)
Article DOI: 10.1038/nature05689
BindingDB Entry DOI: 10.7270/Q2HX1DJ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data