

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50093010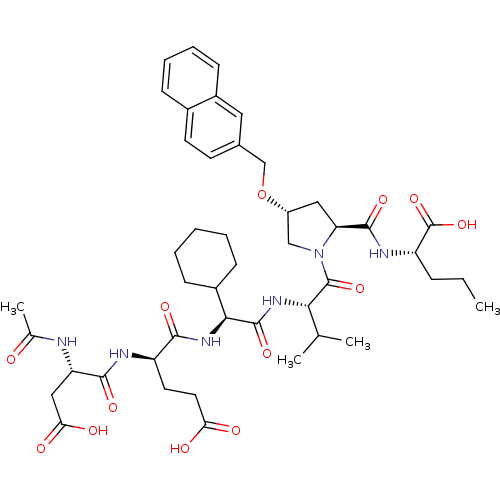 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50029816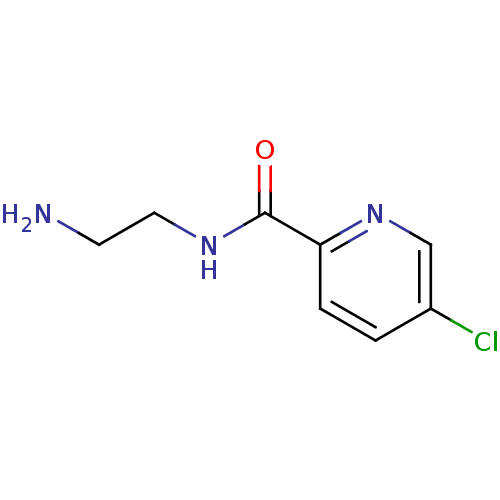 (5-Chloro-pyridine-2-carboxylic acid (2-amino-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187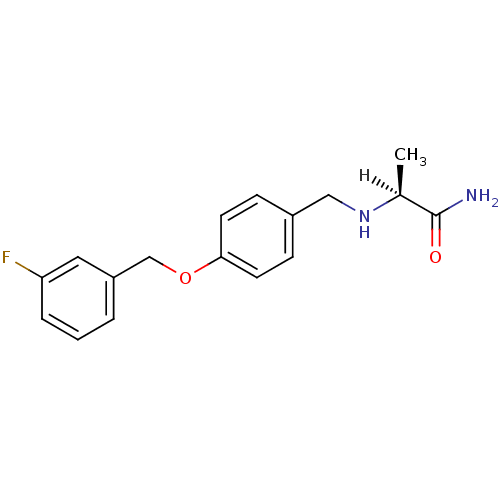 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989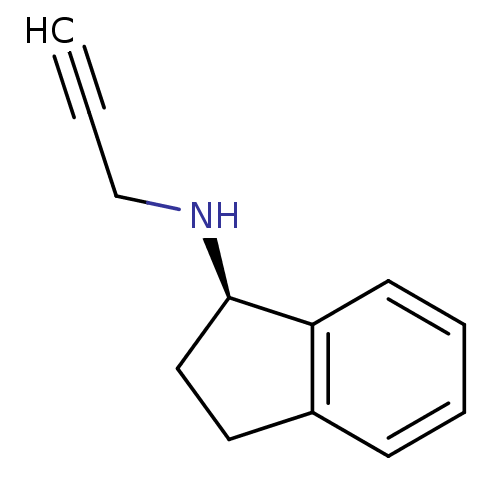 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756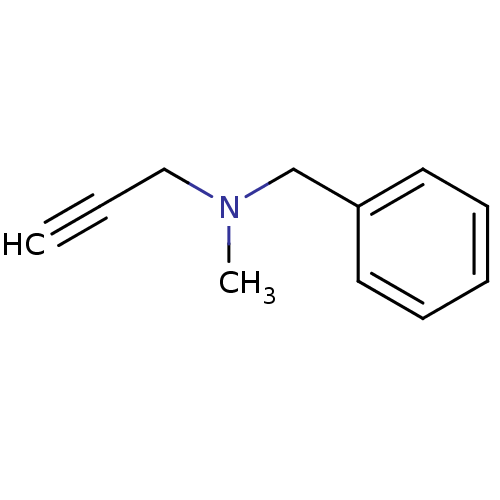 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50063994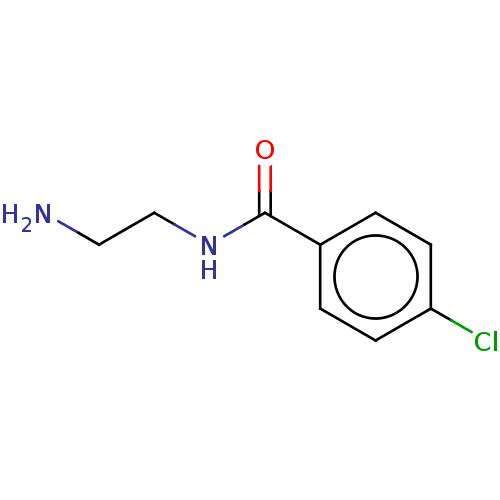 (CHEMBL338404 | Ro-166491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50029816 (5-Chloro-pyridine-2-carboxylic acid (2-amino-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50049240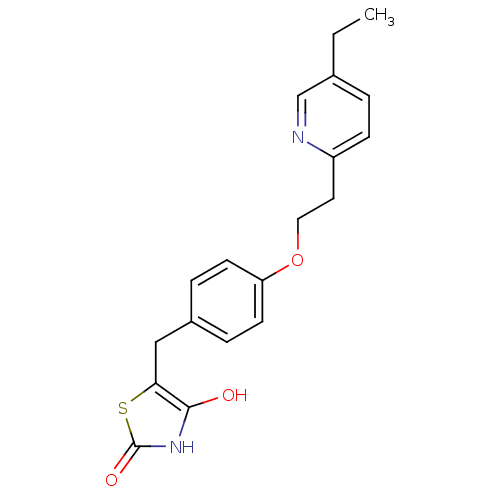 ((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50037429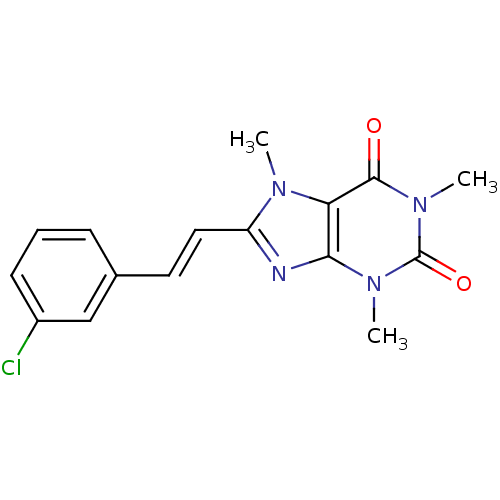 (8-(3-chlorostyryl)caffeine | 8-[(E)-2-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50049240 ((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071982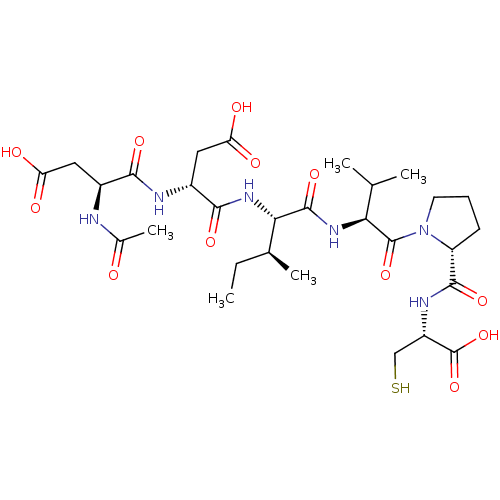 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50070797 (CHEMBL2370476 | Hexapeptide analogue) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50082313 (4-(4-Azido-phenyl)-N-({[3-((Z)-7-oxo-7-{N'-[5-((3a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Supérieure de Chimie de Paris Curated by ChEMBL | Assay Description Binding affinity to human platelet TXA2 receptors as ability to displace binding [3H]-SQ-29,548 | Bioorg Med Chem Lett 9: 2963-8 (1999) BindingDB Entry DOI: 10.7270/Q23J3C50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50063994 (CHEMBL338404 | Ro-166491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 997 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50366517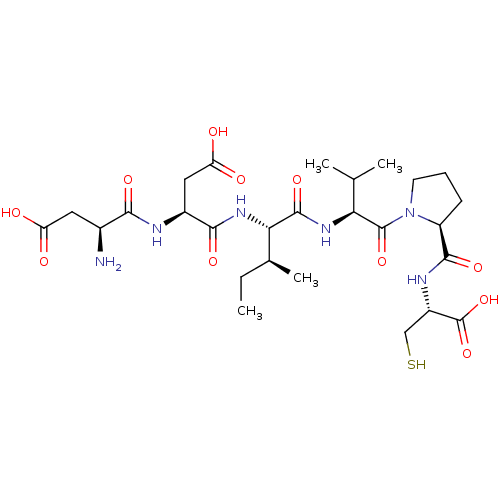 (CHEMBL1790303) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat whole brain homogenate in presence of clorgyline | Bioorg Med Chem 23: 770-8 (2015) Article DOI: 10.1016/j.bmc.2014.12.063 BindingDB Entry DOI: 10.7270/Q2J38V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457704 (CHEMBL4202565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164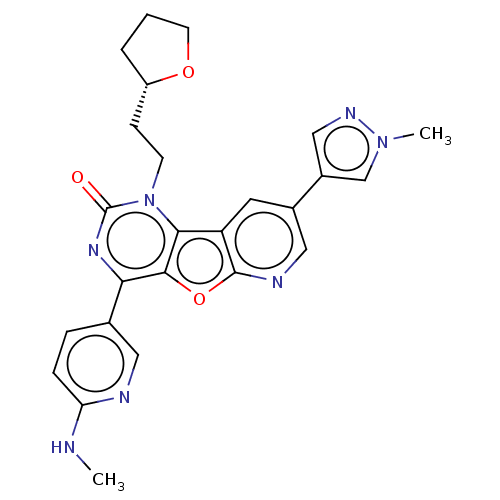 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156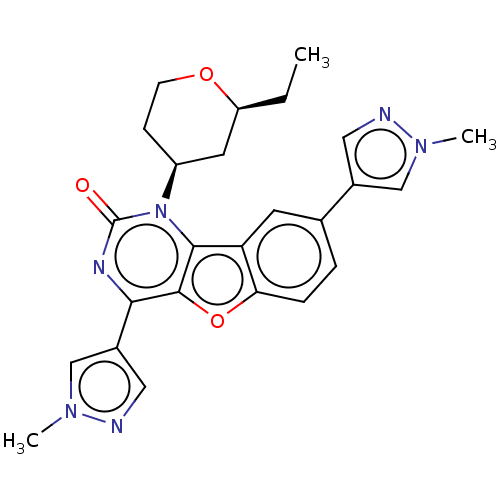 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155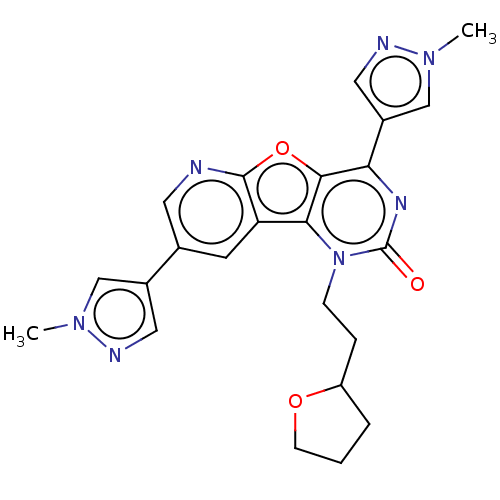 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457706 (CHEMBL4216391) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457693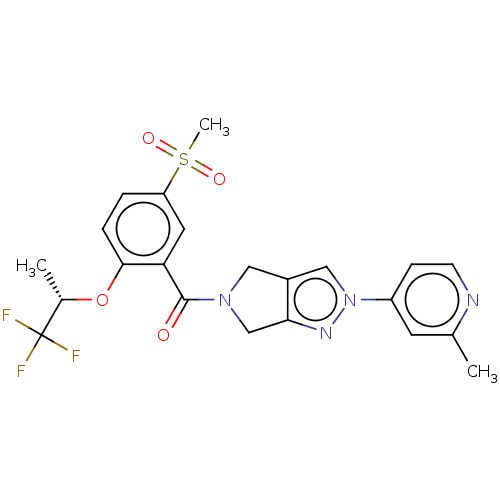 (CHEMBL4218366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176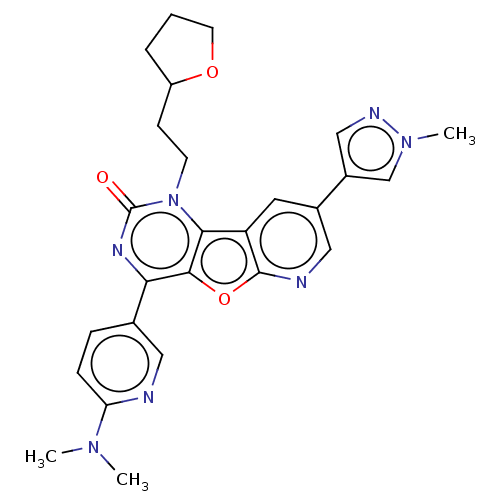 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492175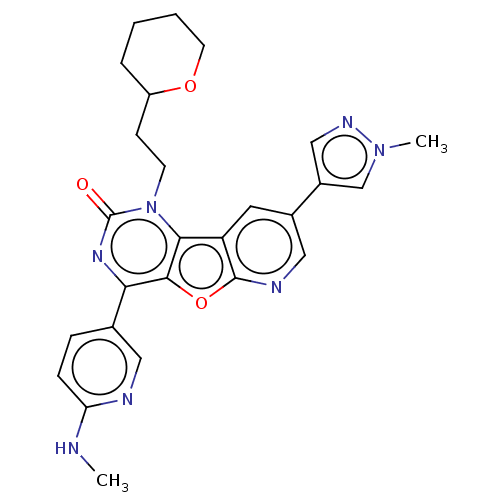 (CHEMBL2397583) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492163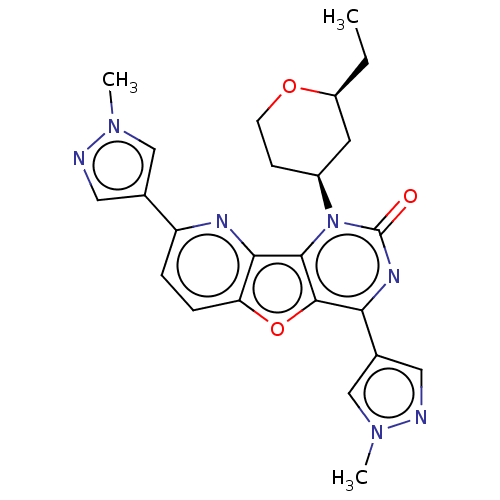 (CHEMBL2397557) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492154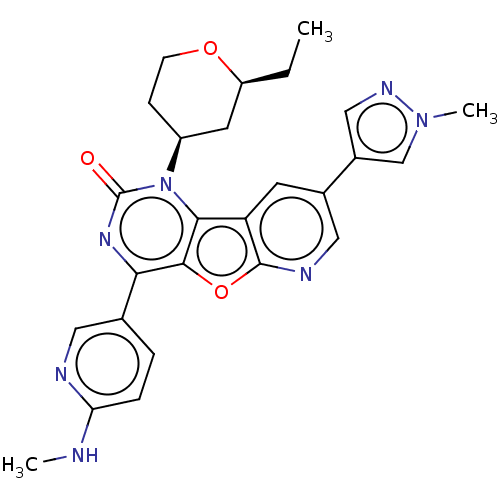 (CHEMBL2397572) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492168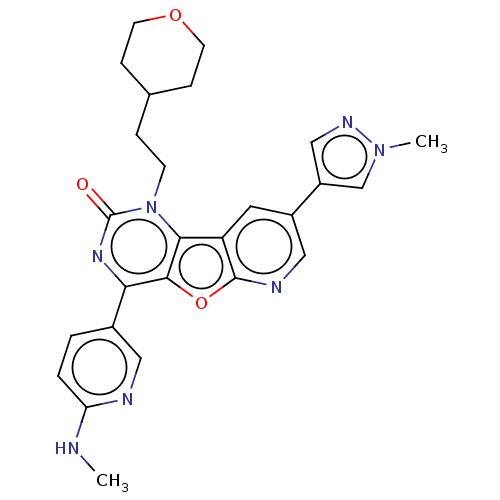 (CHEMBL2397584) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50457704 (CHEMBL4202565) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human GlyT1c expressed in HEK293 cells assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation ... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457695 (CHEMBL4217712) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457692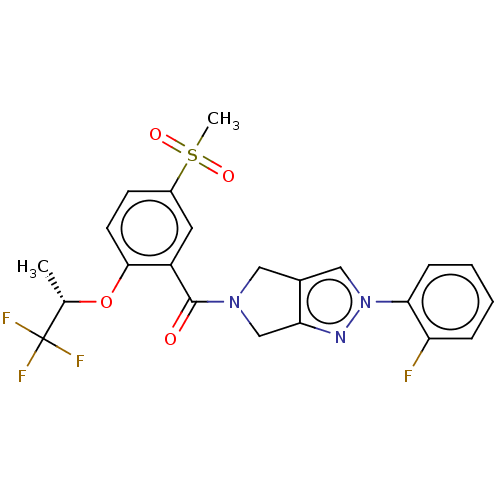 (CHEMBL4211012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457685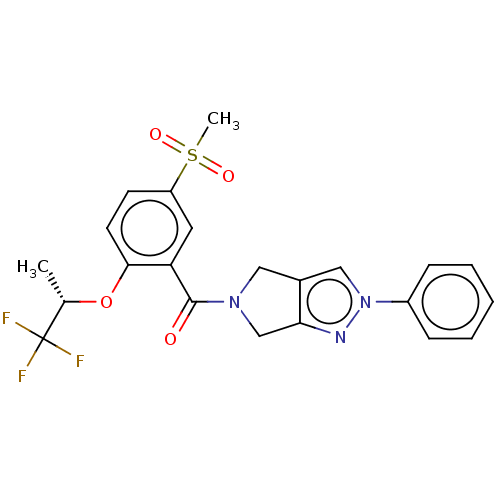 (CHEMBL4210484) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457694 (CHEMBL4211535) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50457689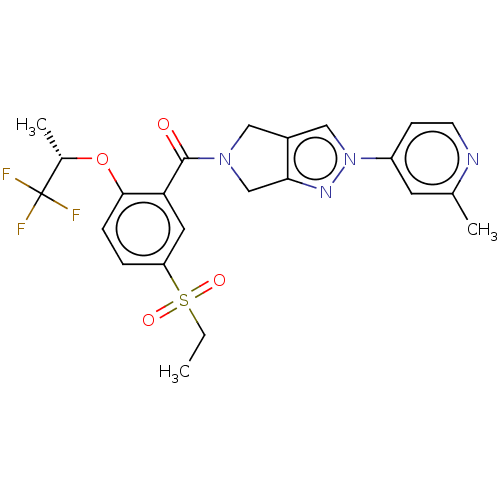 (CHEMBL4206403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience LLC Curated by ChEMBL | Assay Description Inhibition of GlyT1 in primary rat cortical neurons assessed as reduction in [14C]glycine uptake after 2 hrs by microbeta scintillation counting meth... | J Med Chem 61: 6018-6033 (2018) Article DOI: 10.1021/acs.jmedchem.8b00372 BindingDB Entry DOI: 10.7270/Q2GB26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492153 (CHEMBL2397563) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492174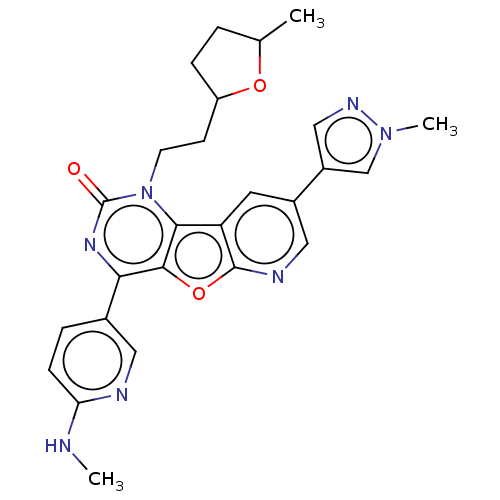 (CHEMBL2397586) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492169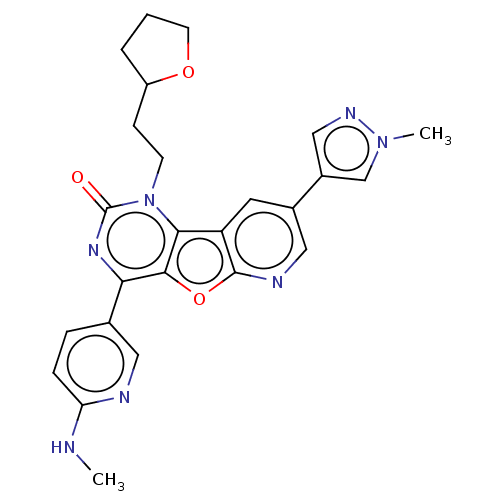 (CHEMBL2397582) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492179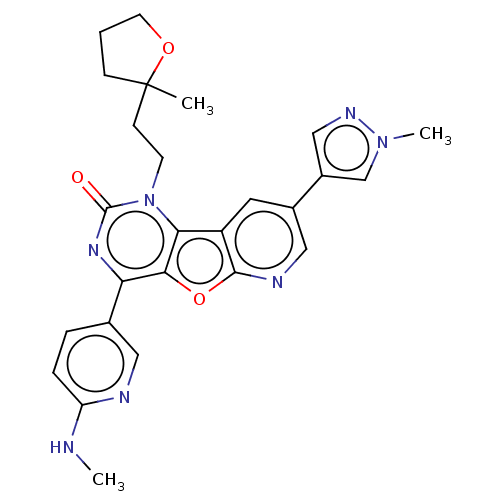 (CHEMBL2397587) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50543922 (CHEMBL4640428) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience Curated by ChEMBL | Assay Description Inhibition of GlyT1 in rat primary cortical neurons assessed as reduction in radiolabeled glycine uptake by scintillation proximity assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127214 BindingDB Entry DOI: 10.7270/Q29027C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492152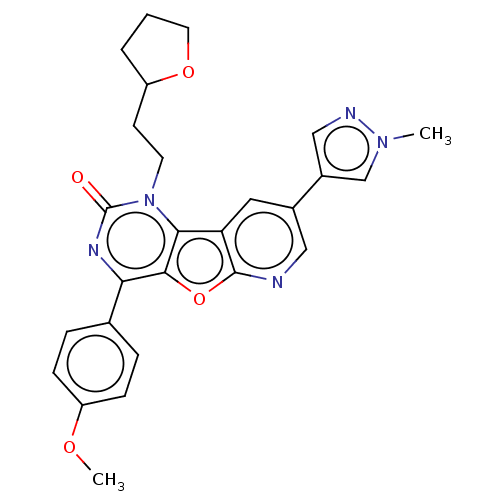 (CHEMBL2397565) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492151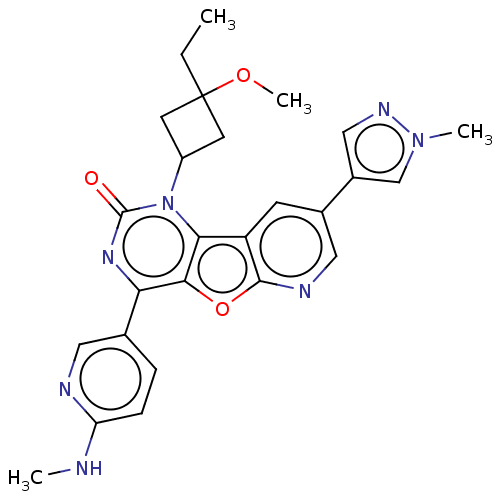 (CHEMBL2397588) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492162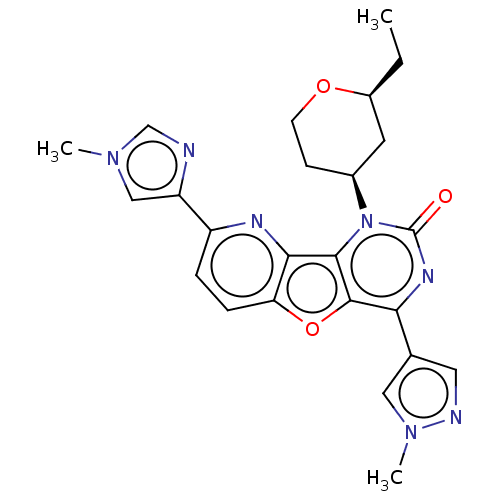 (CHEMBL2397549) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492173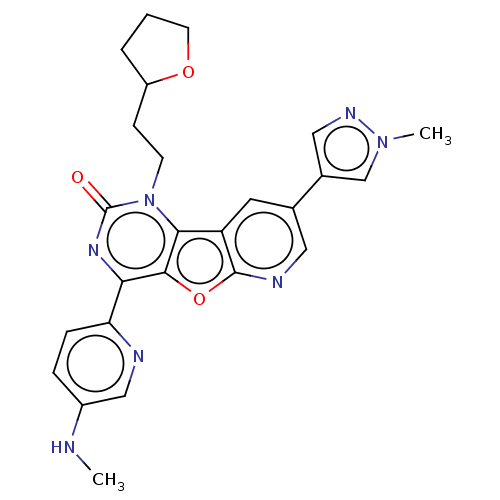 (CHEMBL2397573) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492158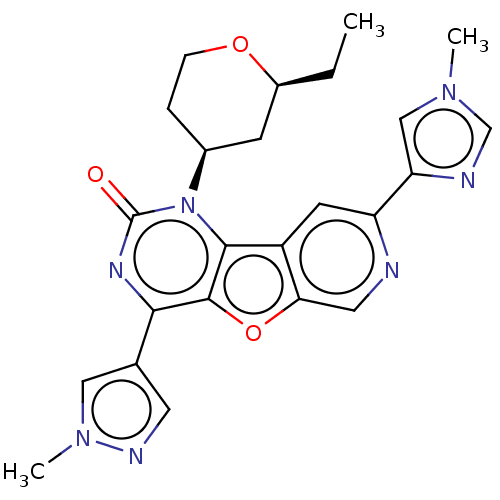 (CHEMBL2397548) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 696 total ) | Next | Last >> |