

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50041517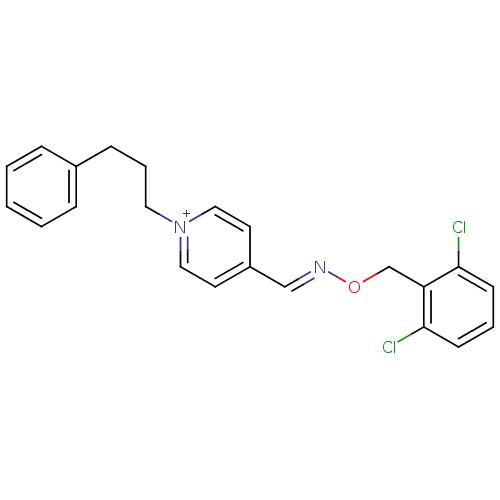 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307921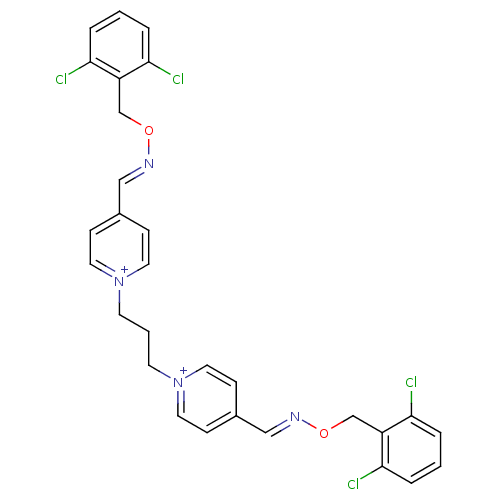 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438825 (CHEMBL2413741) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438843 (CHEMBL2413743) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438830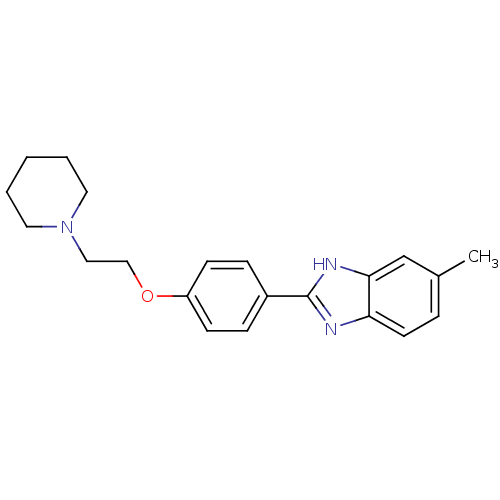 (CHEMBL2413736) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438836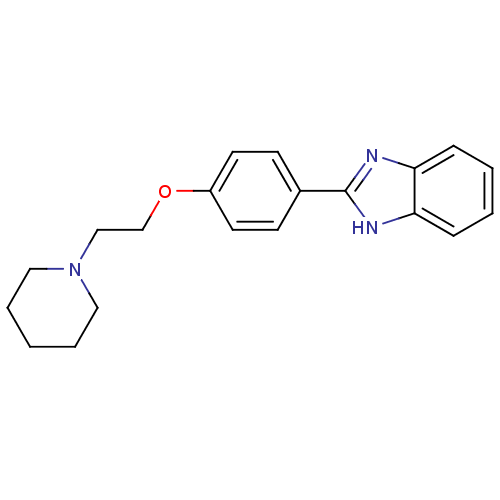 (CHEMBL2413560) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438838 (CHEMBL2413558) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438831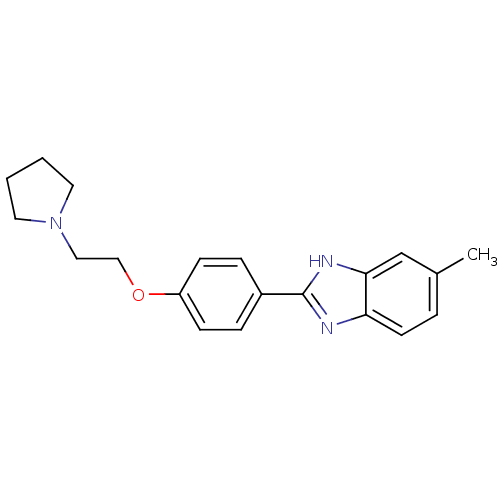 (CHEMBL2413735) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438830 (CHEMBL2413736) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307921 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438832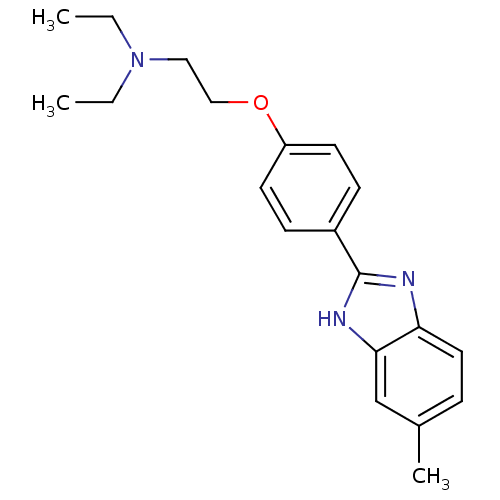 (CHEMBL2413734) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438836 (CHEMBL2413560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438827 (CHEMBL2413739) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50041517 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438837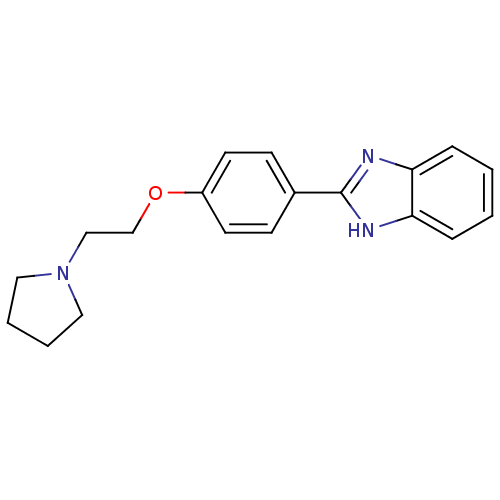 (CHEMBL2413559) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438831 (CHEMBL2413735) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438844 (CHEMBL2413742) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438844 (CHEMBL2413742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438826 (CHEMBL2413740) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438838 (CHEMBL2413558) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438841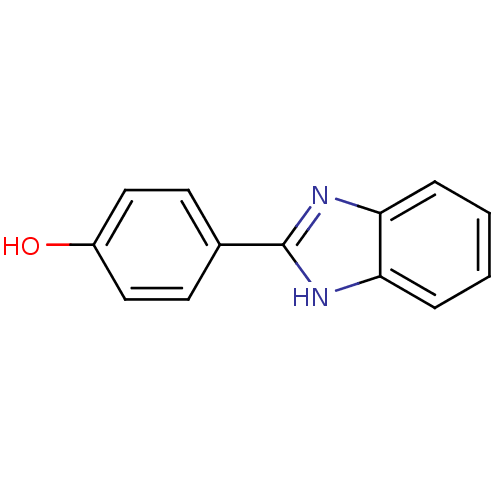 (CHEMBL377740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438834 (CHEMBL2413732) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438839 (CHEMBL2413745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438833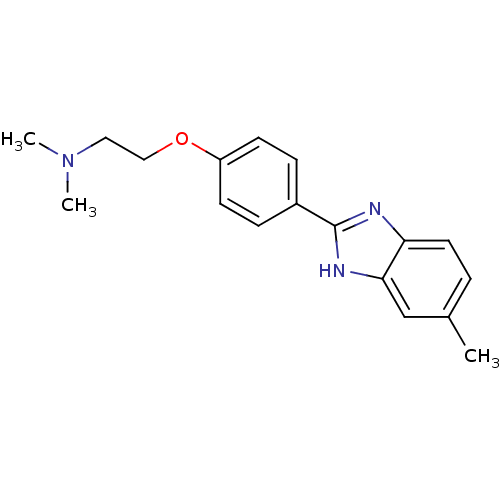 (CHEMBL2413733) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438840 (CHEMBL2413746) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307928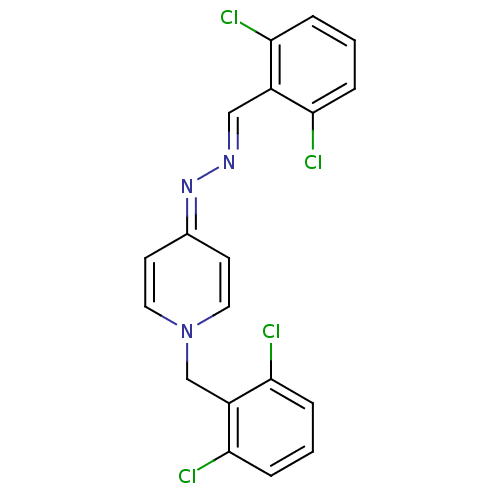 (1-(2,6-dichlorobenzyl)-4-((2,6-dichlorobenzylidene...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438832 (CHEMBL2413734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438825 (CHEMBL2413741) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438828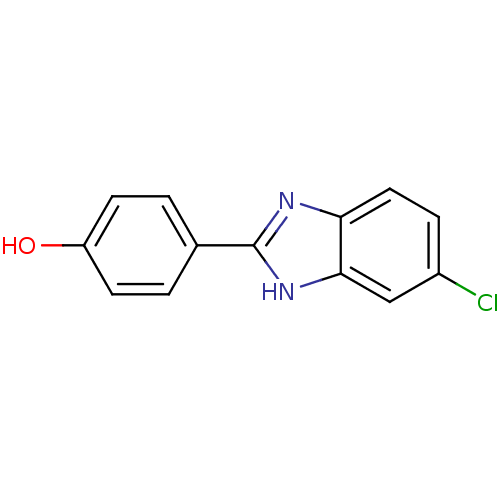 (CHEMBL2413738) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307926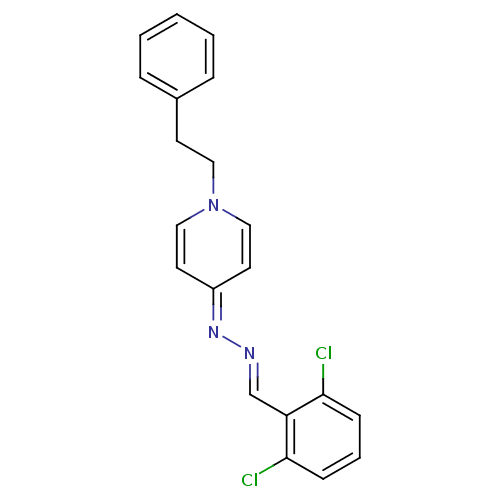 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307924 (4-((2,6-dichlorobenzylidene)hydrazono)-1-methyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438842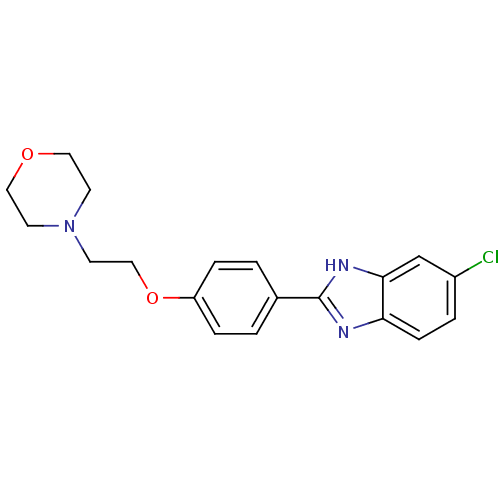 (CHEMBL2413744) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231232 (1-(2-phenylpropyl)-4-oxopiperidine O-(2,6-Dichloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438840 (CHEMBL2413746) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438828 (CHEMBL2413738) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438839 (CHEMBL2413745) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438834 (CHEMBL2413732) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438841 (CHEMBL377740) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438835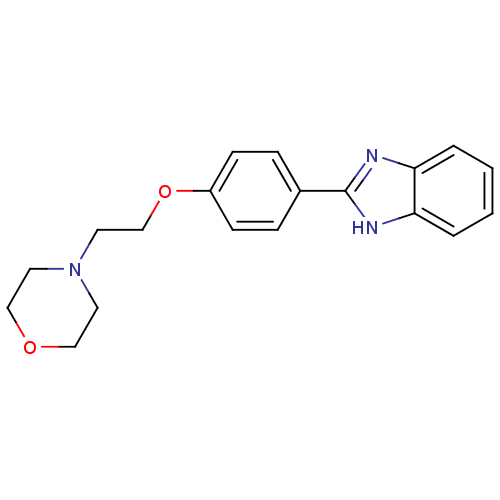 (CHEMBL2413561) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231234 (1-(3-phenylpropyl)piperidine-4-carbaldehyde O-2,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438837 (CHEMBL2413559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307926 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438833 (CHEMBL2413733) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50438829 (CHEMBL2413737) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method | Bioorg Med Chem 21: 4928-37 (2013) Article DOI: 10.1016/j.bmc.2013.06.065 BindingDB Entry DOI: 10.7270/Q21837XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |