

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001974 (6-Dipropylamino-1,2,6,7-tetrahydro-5H-pyrido[3,2,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001969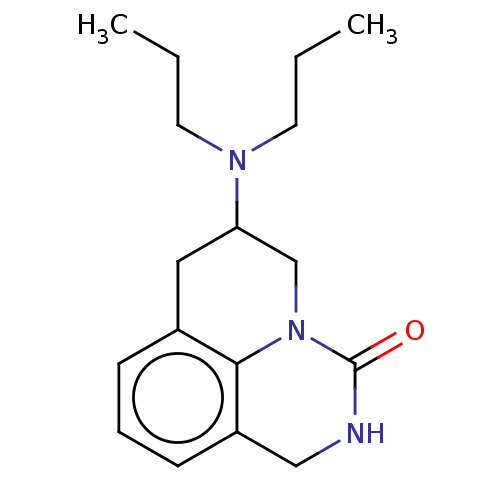 (6-Dipropylamino-1,2,6,7-tetrahydro-5H-pyrido[3,2,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001859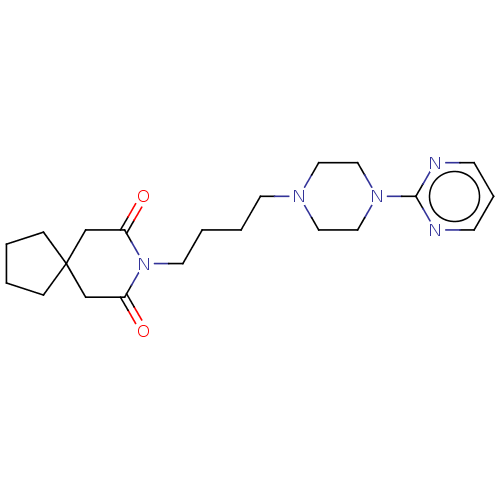 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368591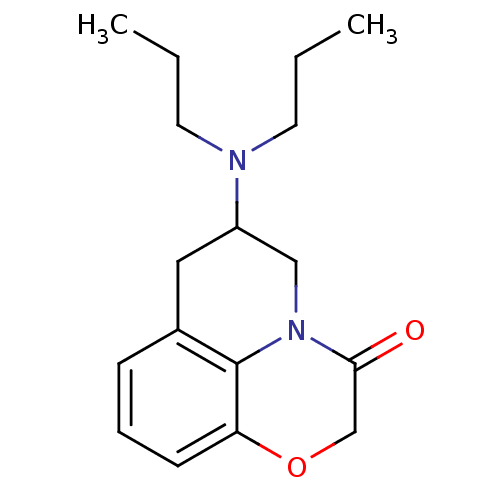 (CHEMBL1794795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368582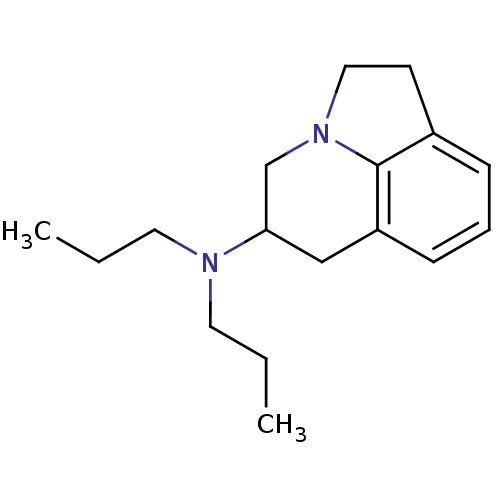 (CHEMBL1794997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001977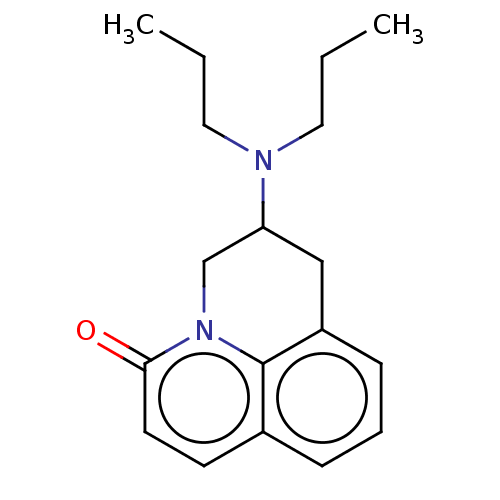 (6-Dipropylamino-6,7-dihydro-5H-pyrido[3,2,1-ij]qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368584 (CHEMBL1202415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368593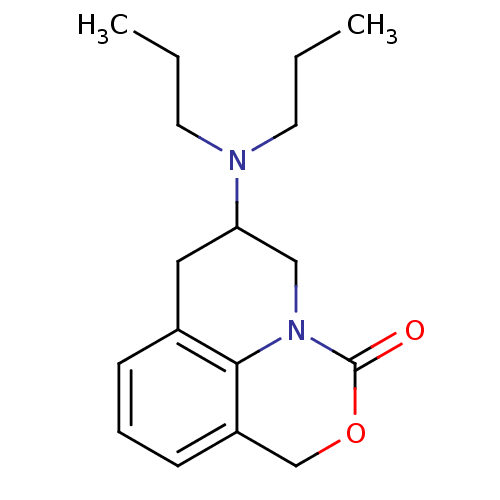 (CHEMBL1202416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368594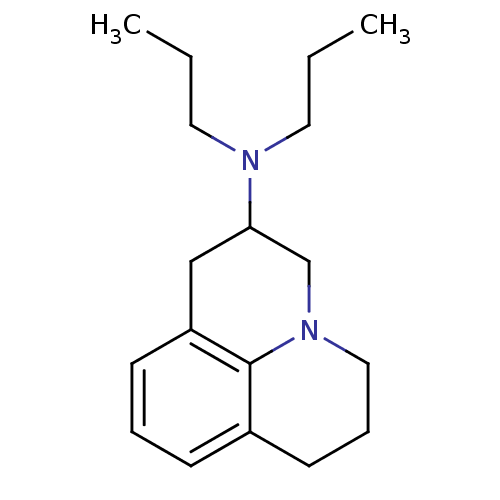 (CHEMBL1794998) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001979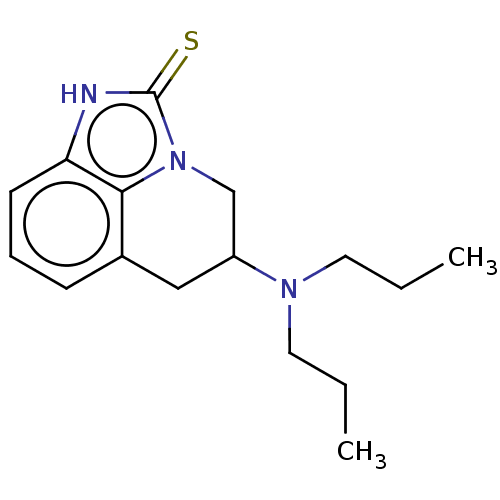 (5-Dipropylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-ij...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368590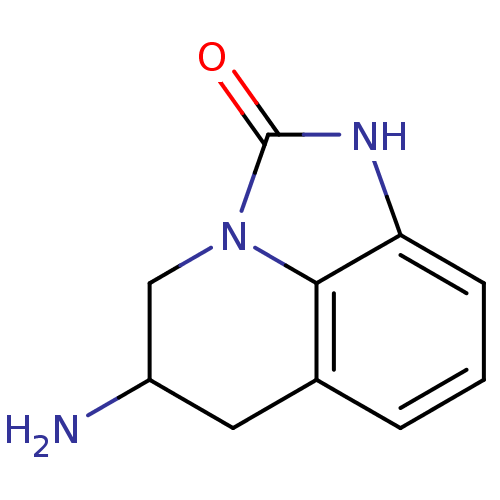 (CHEMBL1202409) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001971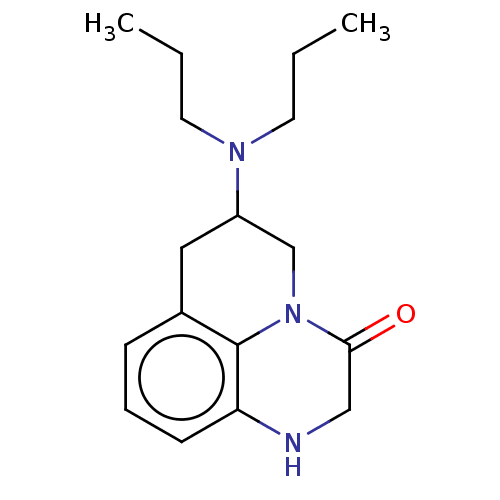 (6-Dipropylamino-1,2,6,7-tetrahydro-5H-pyrido[1,2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM84637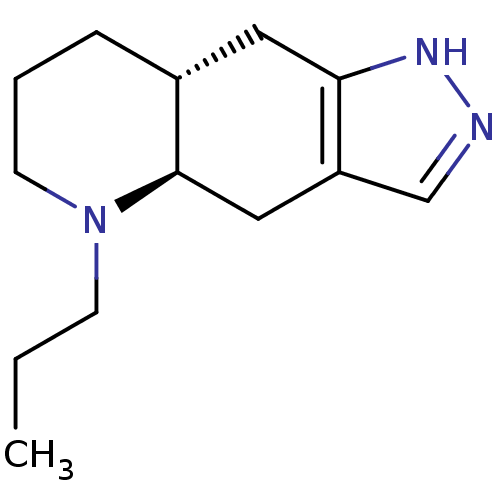 (CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368595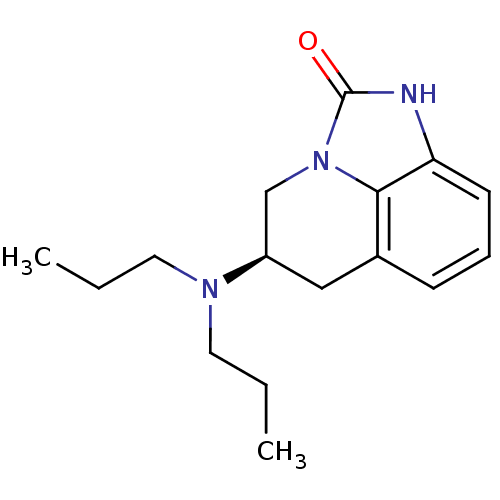 (CHEMBL1744080) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001971 (6-Dipropylamino-1,2,6,7-tetrahydro-5H-pyrido[1,2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001974 (6-Dipropylamino-1,2,6,7-tetrahydro-5H-pyrido[3,2,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368592 (CHEMBL1202412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001979 (5-Dipropylamino-5,6-dihydro-1H,4H-imidazo[4,5,1-ij...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368595 (CHEMBL1744080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368588 (CHEMBL1202413) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001964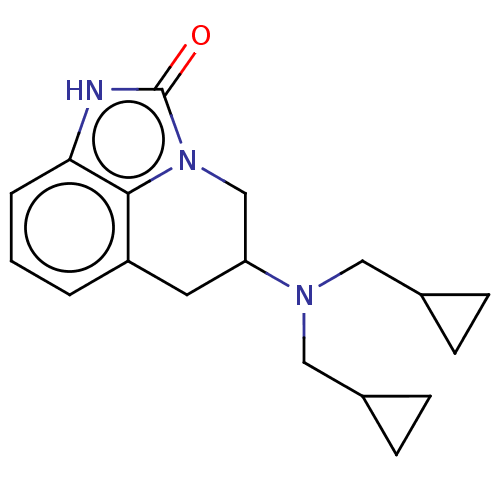 (5-(Bis-cyclopropylmethyl-amino)-5,6-dihydro-1H,4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368587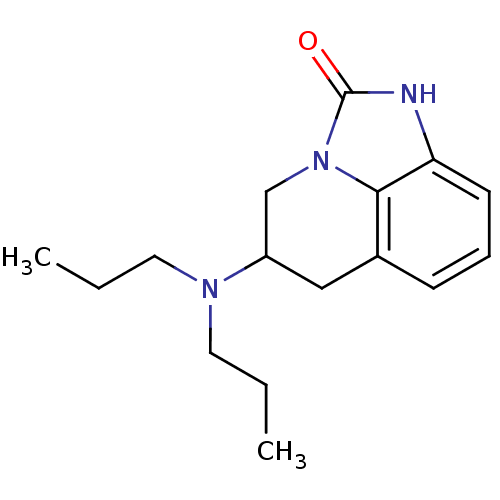 (CHEMBL1202407) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368594 (CHEMBL1794998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001976 (5-Dipropylamino-5,6-dihydro-1H,4H-pyrrolo[3,2,1-ij...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368586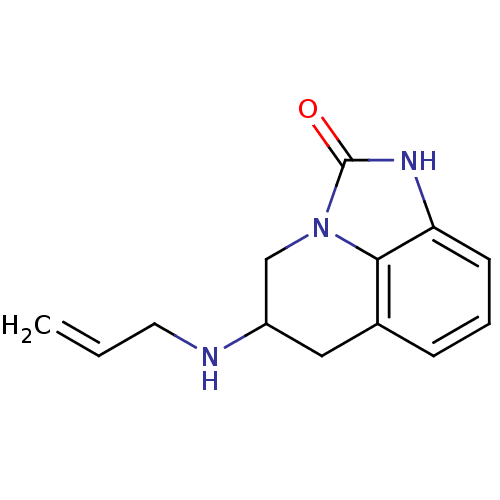 (CHEMBL1202410) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001972 (5-Dipropylamino-1-methyl-5,6-dihydro-1H,4H-imidazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]DPAT from 5-HT1A receptor in homogenates of bovine hippocampus | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368596 (CHEMBL1202414) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001978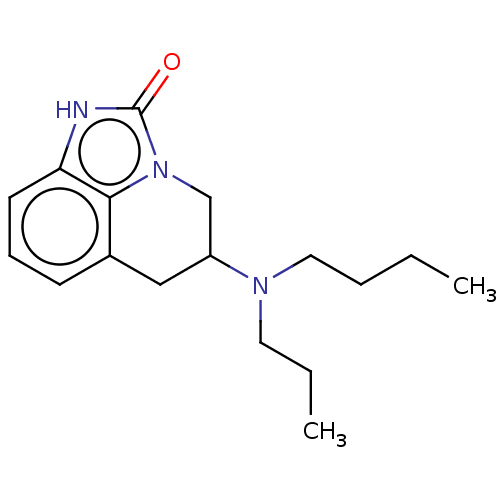 (5-(Butyl-propyl-amino)-5,6-dihydro-1H,4H-imidazo[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001978 (5-(Butyl-propyl-amino)-5,6-dihydro-1H,4H-imidazo[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001958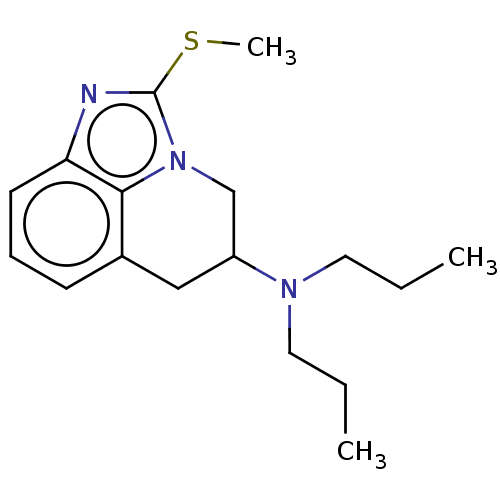 ((2-Methylsulfanyl-5,6-dihydro-4H-imidazo[4,5,1-ij]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368587 (CHEMBL1202407) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368583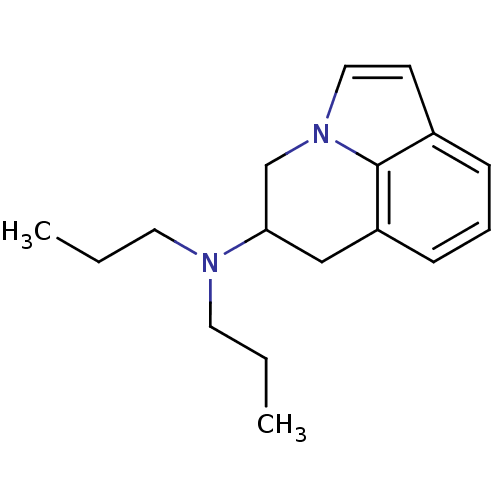 (CHEMBL1794999) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368583 (CHEMBL1794999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368593 (CHEMBL1202416) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 292 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368584 (CHEMBL1202415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001977 (6-Dipropylamino-6,7-dihydro-5H-pyrido[3,2,1-ij]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50368589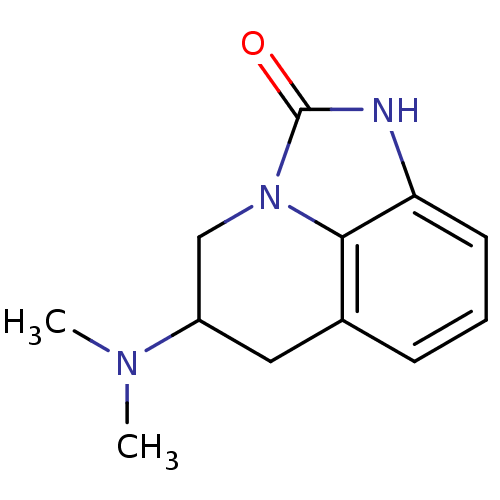 (CHEMBL1202411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368586 (CHEMBL1202410) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 534 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50453287 (CHEMBL2092845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]DPAT from 5-HT1A receptor in homogenates of bovine hippocampus | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001966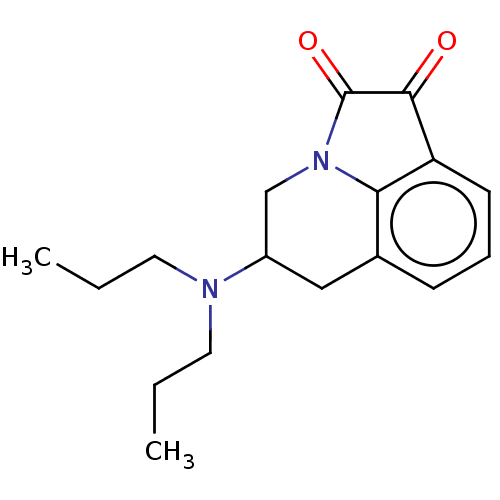 (5-Dipropylamino-5,6-dihydro-4H-pyrrolo[3,2,1-ij]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001962 ((5,6-Dihydro-4H-imidazo[4,5,1-ij]quinolin-5-yl)-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 827 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001957 (5-Dipropylamino-5,6-dihydro-4H-oxazolo[5,4,3-ij]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001972 (5-Dipropylamino-1-methyl-5,6-dihydro-1H,4H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 924 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001980 (6-Dipropylamino-6,7-dihydro-5H-pyrido[1,2,3-de]qui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001958 ((2-Methylsulfanyl-5,6-dihydro-4H-imidazo[4,5,1-ij]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368585 (CHEMBL1202408) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]raclopride from Dopamine receptor D2 in rat striatal homogenates | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001966 (5-Dipropylamino-5,6-dihydro-4H-pyrrolo[3,2,1-ij]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]raclopride from dopamine receptor D2 in rat striatal homogenates. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001960 (5-(Benzyl-propyl-amino)-5,6-dihydro-1H,4H-imidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Ability to displace [3H]-DPAT from 5-hydroxytryptamine 1A receptor in homogenates of bovine hippocampus. | J Med Chem 35: 1076-92 (1992) BindingDB Entry DOI: 10.7270/Q2DB82GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |