

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021345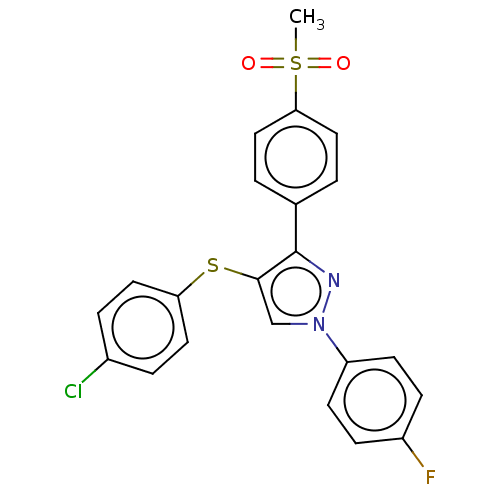 (CHEMBL3287928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021331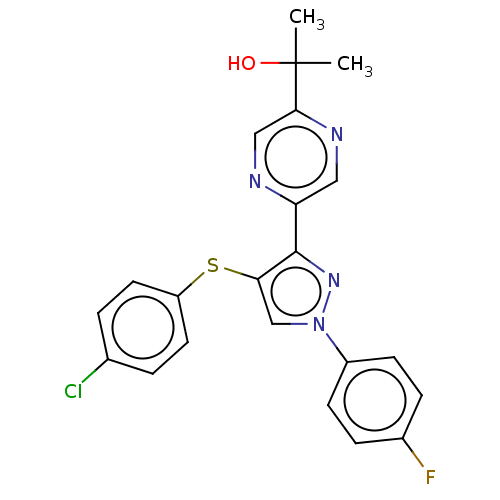 (CHEMBL3287930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50350538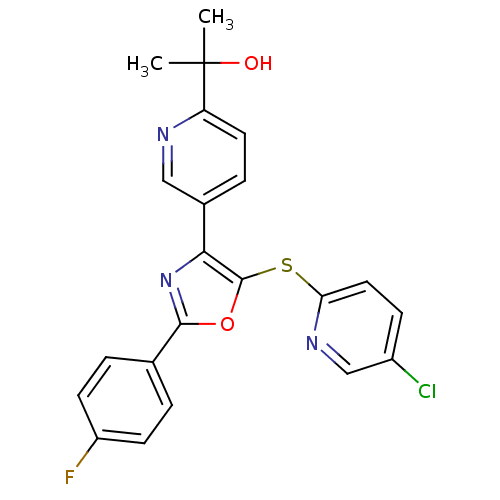 (CHEMBL1812717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021346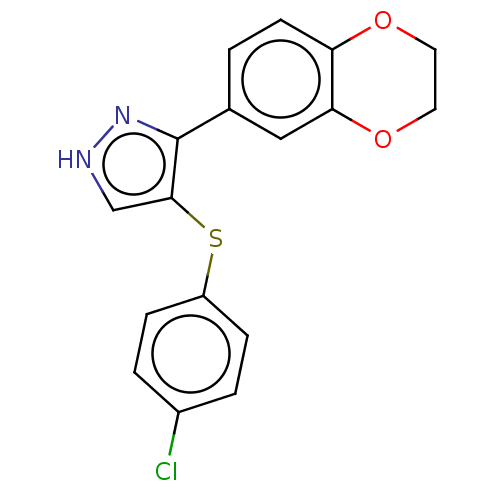 (CHEMBL3287926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021329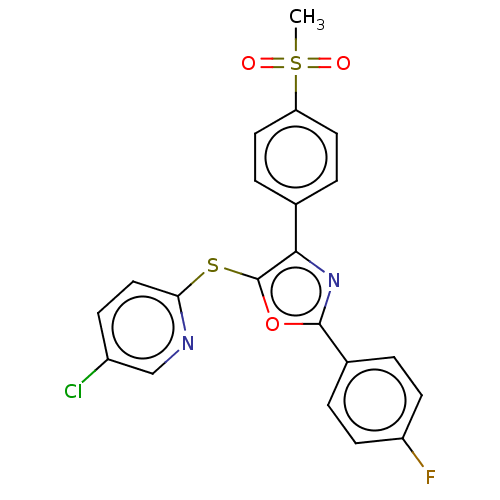 (CHEMBL3287932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021344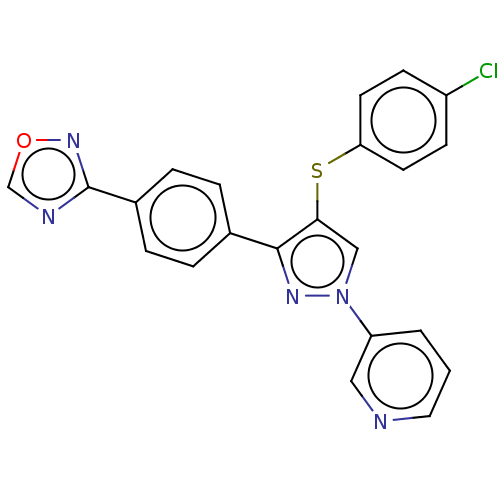 (CHEMBL3287929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021334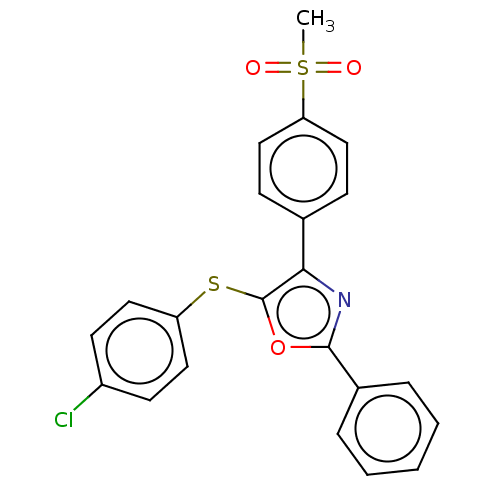 (CHEMBL3287931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50021330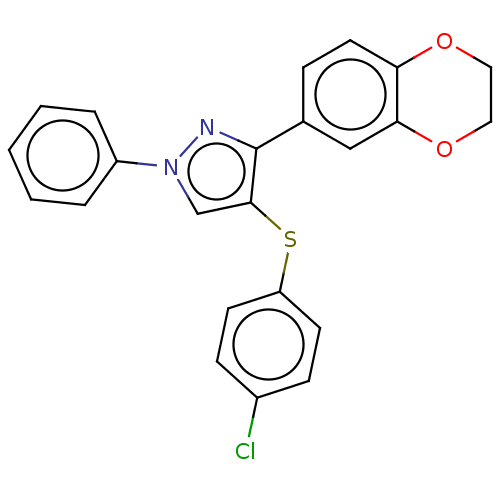 (CHEMBL3287927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [35S]MK-499 from human ERG | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021344 (CHEMBL3287929) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021334 (CHEMBL3287931) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021345 (CHEMBL3287928) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021330 (CHEMBL3287927) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021331 (CHEMBL3287930) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50350538 (CHEMBL1812717) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50350538 (CHEMBL1812717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021344 (CHEMBL3287929) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021334 (CHEMBL3287931) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021345 (CHEMBL3287928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021329 (CHEMBL3287932) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021330 (CHEMBL3287927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071296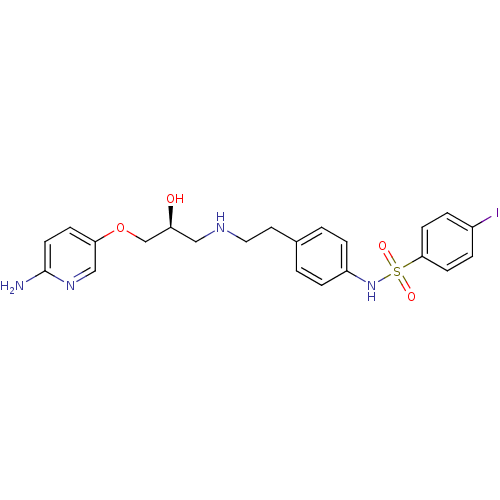 (CHEMBL64201 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021329 (CHEMBL3287932) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021331 (CHEMBL3287930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071291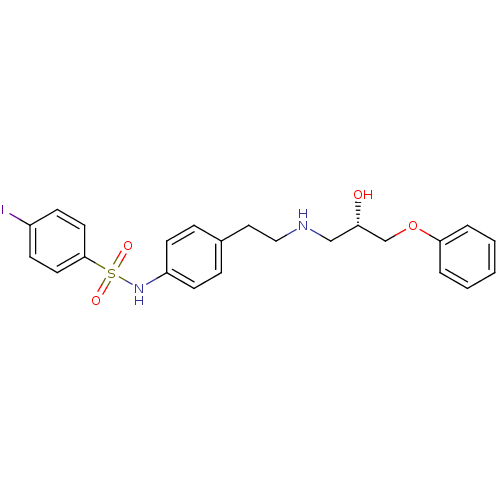 (CHEMBL442172 | N-{4-[2-((S)-2-Hydroxy-3-phenoxy-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071291 (CHEMBL442172 | N-{4-[2-((S)-2-Hydroxy-3-phenoxy-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071300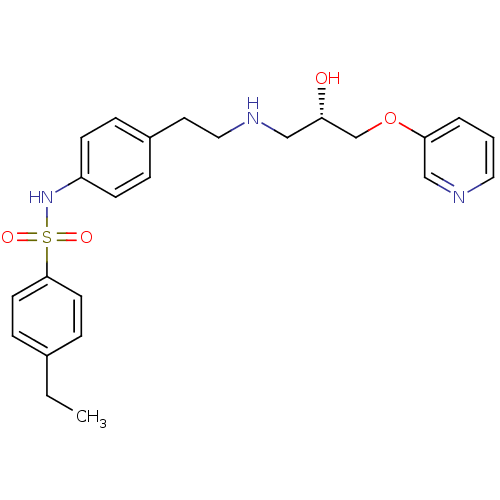 (4-Ethyl-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071278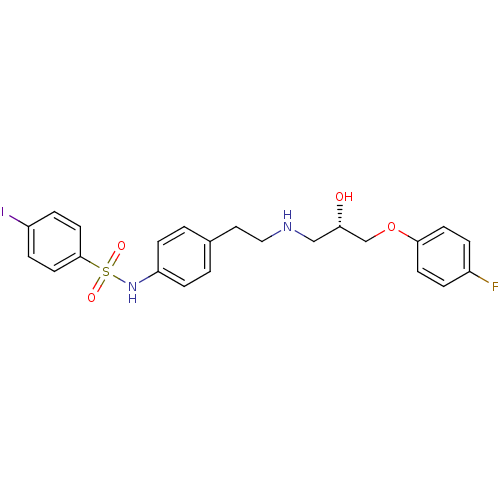 (CHEMBL304878 | N-(4-{2-[(S)-3-(4-Fluoro-phenoxy)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071290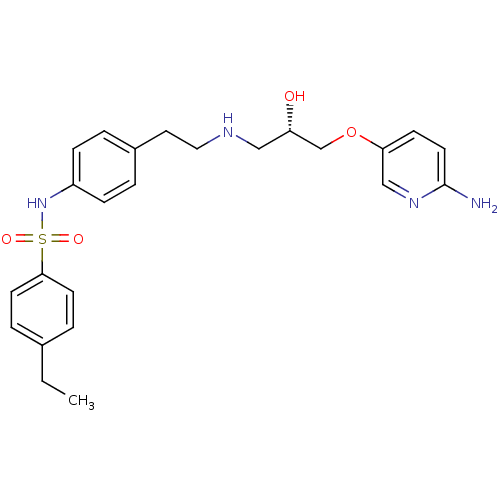 (CHEMBL304090 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071294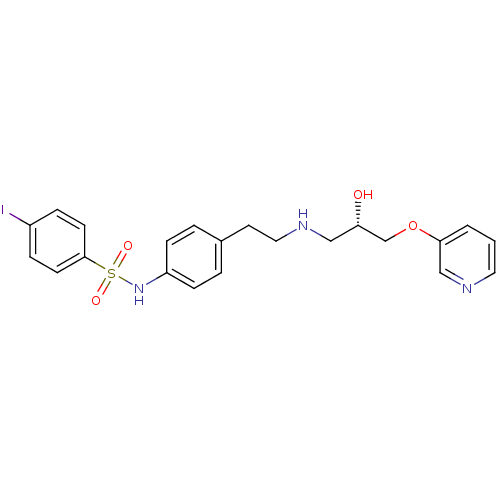 (CHEMBL294042 | L-74372 | N-(4-{2-[(S)-2-Hydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50021346 (CHEMBL3287926) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50070152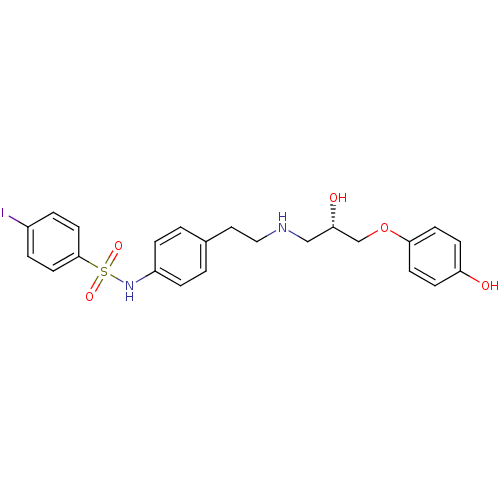 (CHEMBL276257 | N-(4-{2-[(S)-2-Hydroxy-3-(4-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50021346 (CHEMBL3287926) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH lysate using AMCAA as substrate by fluorescence assay | ACS Med Chem Lett 5: 717-21 (2014) Article DOI: 10.1021/ml5001239 BindingDB Entry DOI: 10.7270/Q2JQ12KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071292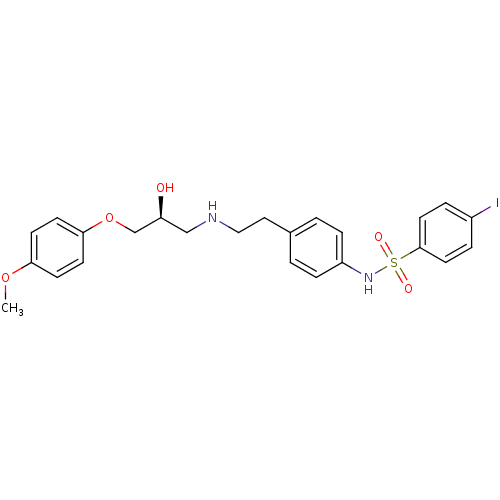 (CHEMBL67886 | N-(4-{2-[(S)-2-Hydroxy-3-(4-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50070152 (CHEMBL276257 | N-(4-{2-[(S)-2-Hydroxy-3-(4-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071285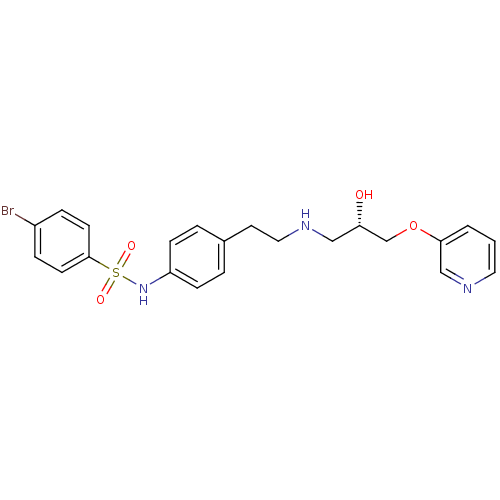 (4-Bromo-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071279 (3,4-Dichloro-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071279 (3,4-Dichloro-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071287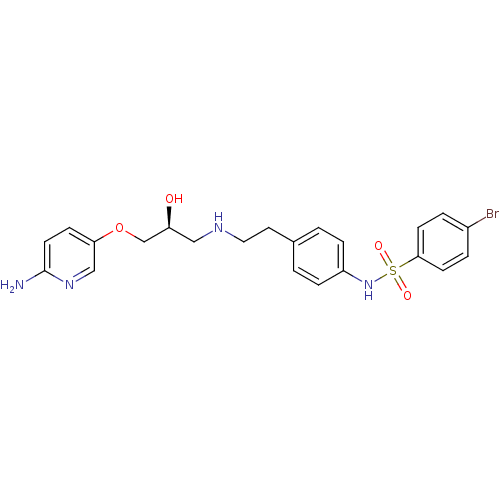 (CHEMBL63842 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071276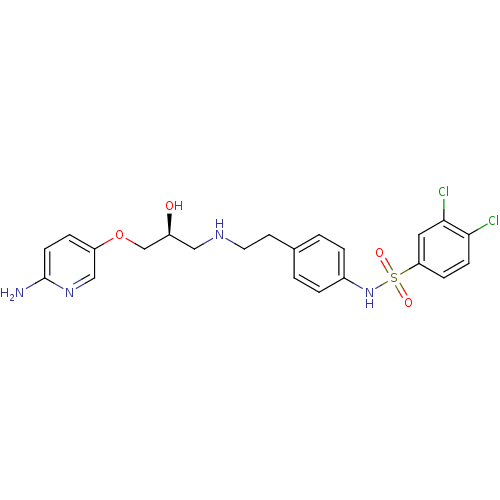 (CHEMBL66859 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50172000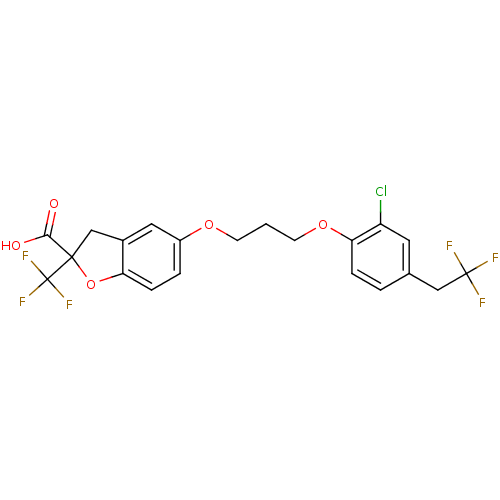 (5-{3-[2-Chloro-4-(2,2,2-trifluoro-ethyl)-phenoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for PPAR-delta | J Med Chem 48: 5589-99 (2005) Article DOI: 10.1021/jm050373g BindingDB Entry DOI: 10.7270/Q25T3K0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071298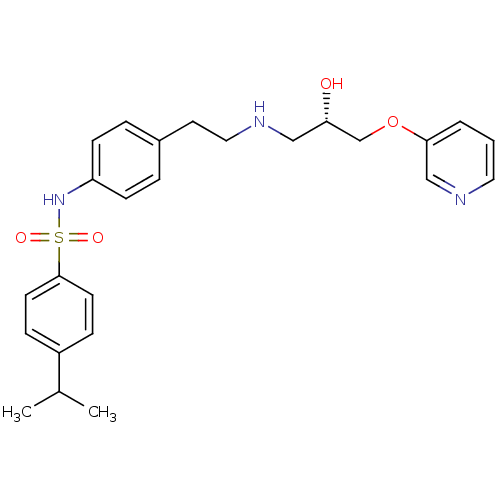 (CHEMBL292947 | N-(4-{2-[(S)-2-Hydroxy-3-(pyridin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071284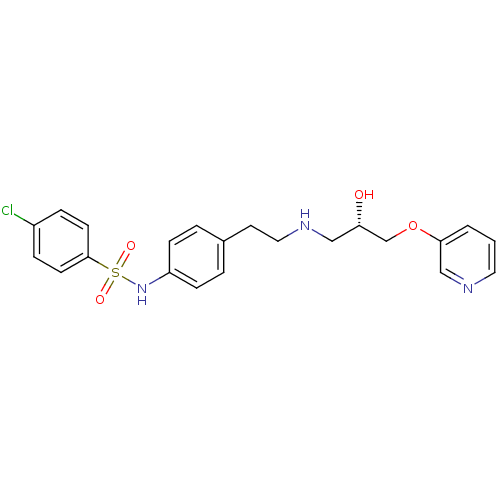 (4-Chloro-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-yloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071293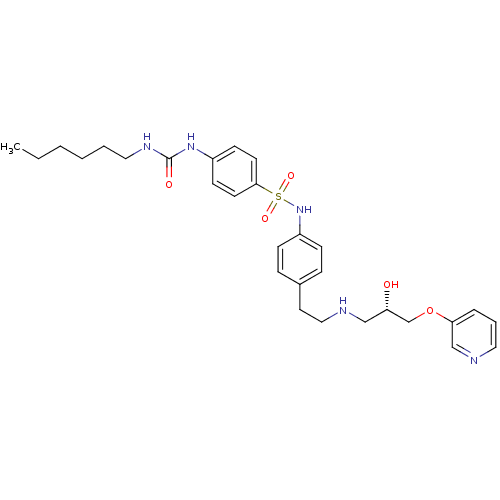 (4-(3-Hexyl-ureido)-N-(4-{2-[(S)-2-hydroxy-3-(pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071290 (CHEMBL304090 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071276 (CHEMBL66859 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071297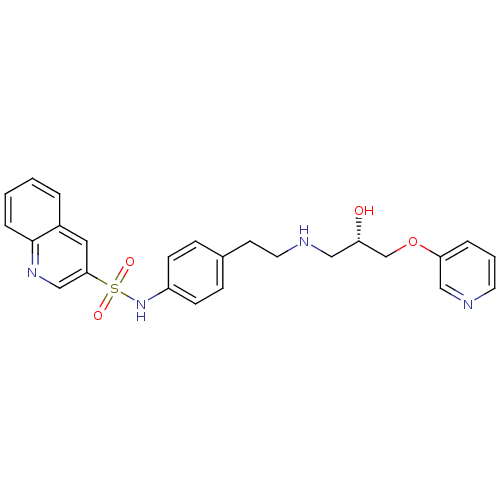 (CHEMBL68334 | Quinoline-3-sulfonic acid (4-{2-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071295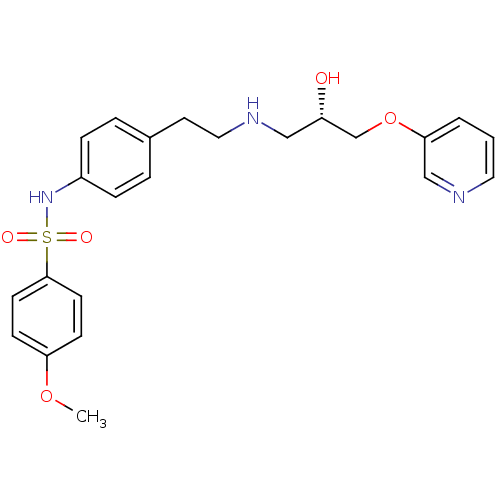 (CHEMBL66964 | N-(4-{2-[(S)-2-Hydroxy-3-(pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50071282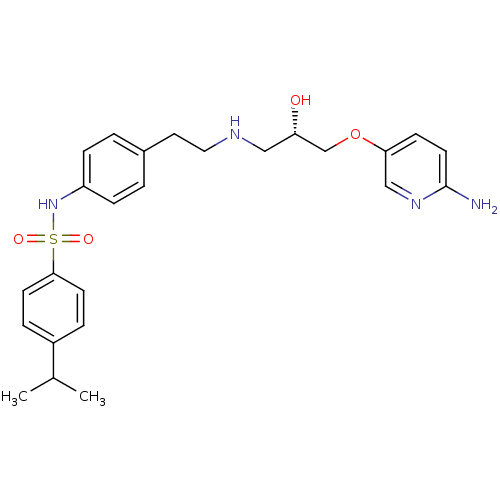 (CHEMBL64760 | N-(4-{2-[(S)-3-(6-Amino-pyridin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-2 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071300 (4-Ethyl-N-(4-{2-[(S)-2-hydroxy-3-(pyridin-3-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50071293 (4-(3-Hexyl-ureido)-N-(4-{2-[(S)-2-hydroxy-3-(pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for its agonist activity and the binding affinity against human Beta-1 adrenergic receptor in membranes from chinese hamster ovary cell | Bioorg Med Chem Lett 8: 2111-6 (1999) BindingDB Entry DOI: 10.7270/Q2H41QKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 210 total ) | Next | Last >> |