

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50112584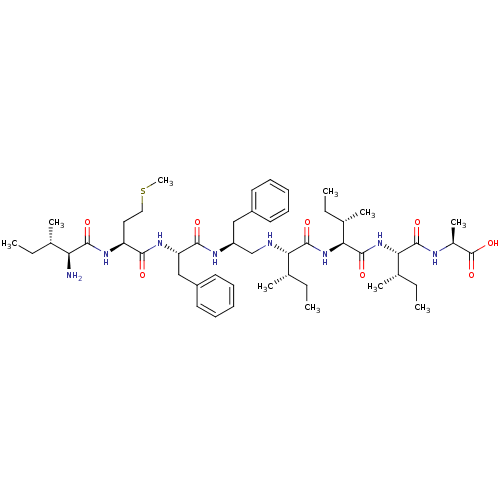 (2-(2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370072 (CHEMBL1790422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112580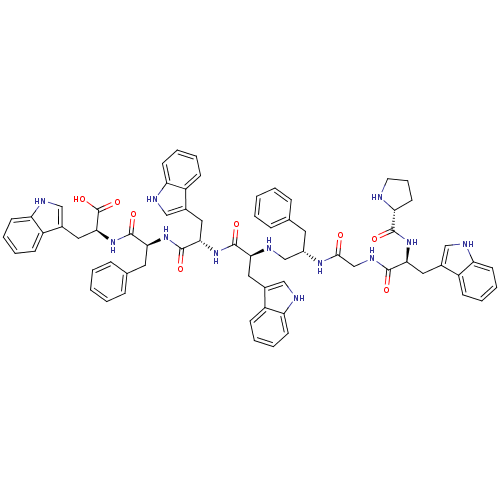 (CHEMBL268148 | PWGF(CH2NH)WWFW) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112597 (2-{2-[2-(2-{2-[2-[2-(2-Amino-3-carboxy-propionylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112581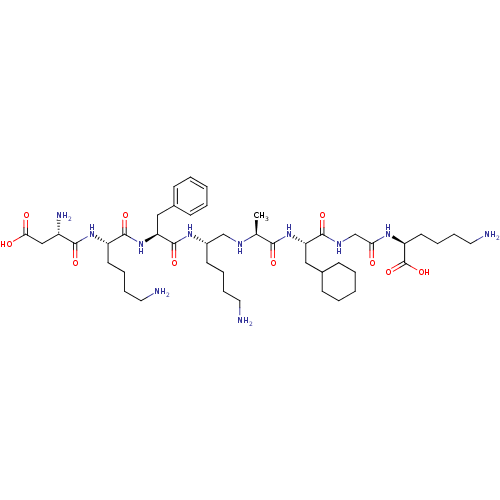 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112596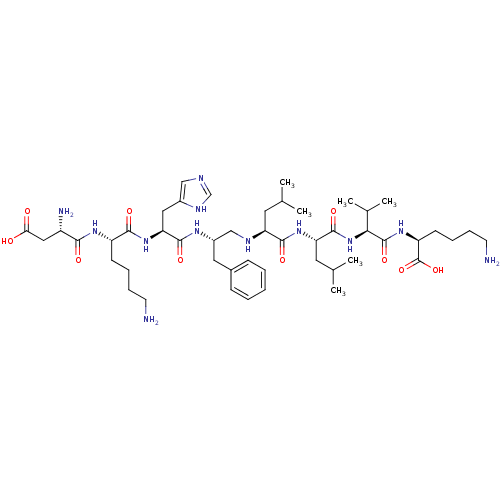 (6-Amino-2-{2-[2-(2-{2-[2-[6-amino-2-(2-amino-3-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112594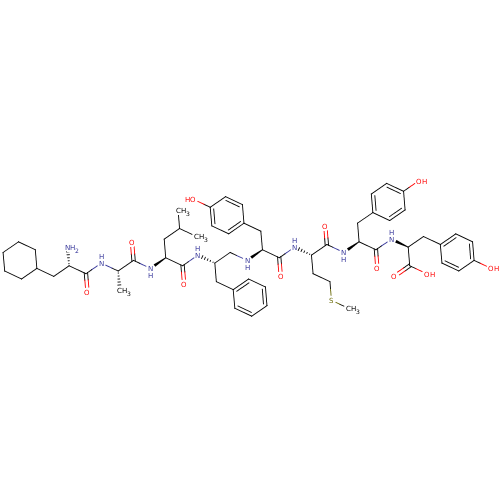 (CHEMBL266692 | ChaALF(CH2NH)YMYY) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112583 (1-{2-[2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112579 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[2-(2-amino-3-cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112591 (2-(2-{2-[2-(2-{2-[2-(2-Amino-4-methylsulfanyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112585 (2-[3-Cyclohexyl-2-(2-{2-[2-(3-(3H-imidazol-4-yl)-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112582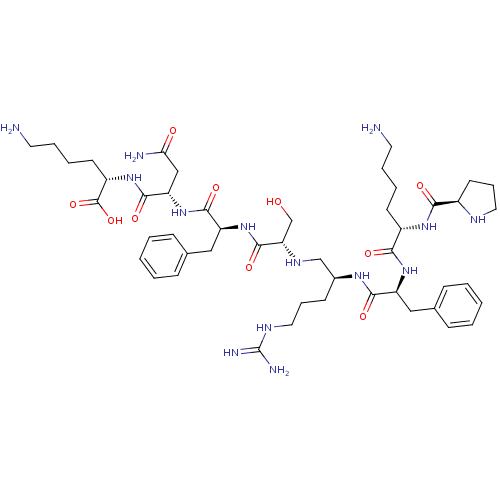 (6-Amino-2-[2-(2-{2-[2-(2-{6-amino-2-[(pyrrolidine-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370073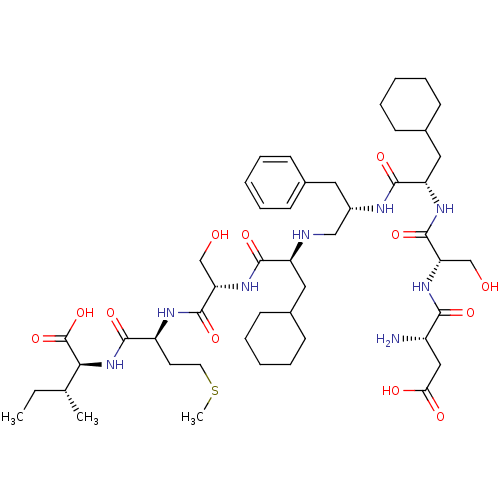 (CHEMBL1790421) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370068 (CHEMBL1790423) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370070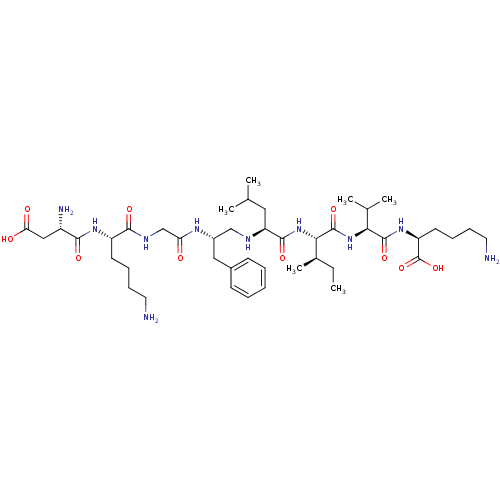 (CHEMBL1790419) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370074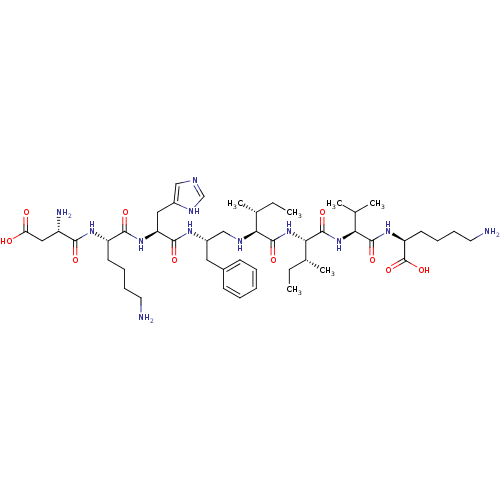 (CHEMBL1790420) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370069 (CHEMBL1790418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112592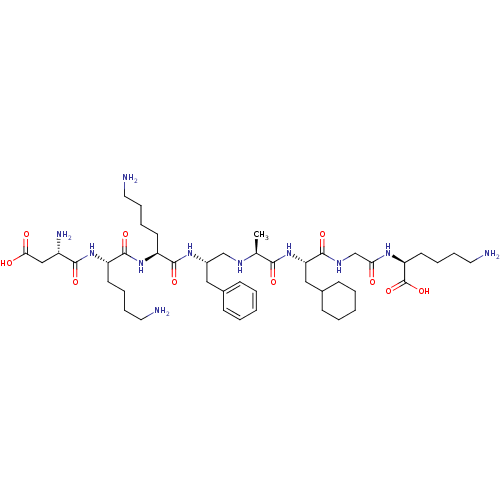 (6-Amino-2-(2-{2-[2-(2-{6-amino-2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112587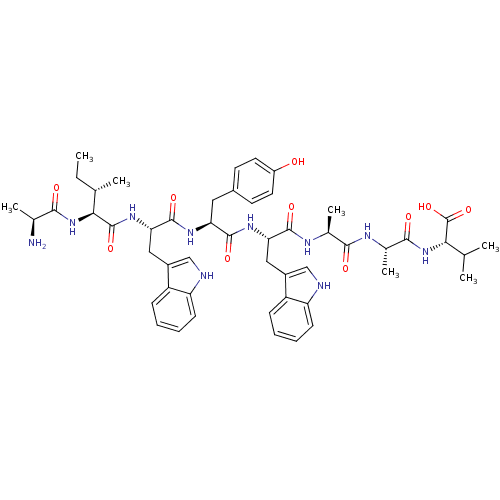 (2-(2-{2-[2-[2-[2-[2-(2-Amino-propionylamino)-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370071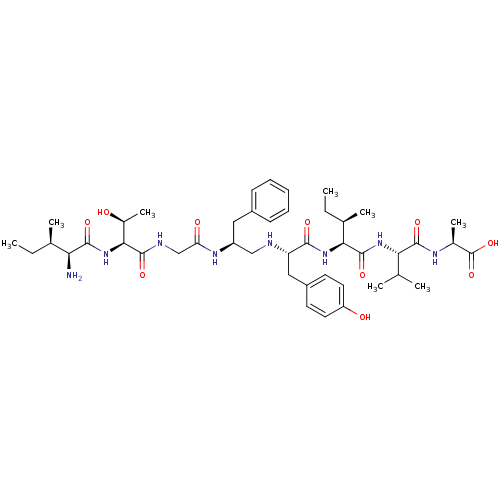 (CHEMBL1790424) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112588 (2-[2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-butyrylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112578 (2-(2-{2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50378032 (CHEMBL452483 | Guttiferone A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50378032 (CHEMBL452483 | Guttiferone A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50378032 (CHEMBL452483 | Guttiferone A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin G by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50249351 (CHEMBL470519 | epiclusianone) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50249351 (CHEMBL470519 | epiclusianone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin G by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50249351 (CHEMBL470519 | epiclusianone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50249352 (CHEMBL470520 | garciniaphenone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin G by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50249352 (CHEMBL470520 | garciniaphenone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50249352 (CHEMBL470520 | garciniaphenone) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50249353 (1,1,3-trihydroxybenzophenone | 2,2',4-trihydroxybe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM22726 (Benzophenone | CHEMBL90039 | diphenylmethanone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin B by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50249353 (1,1,3-trihydroxybenzophenone | 2,2',4-trihydroxybe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of cathepsin G by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50249353 (1,1,3-trihydroxybenzophenone | 2,2',4-trihydroxybe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM22726 (Benzophenone | CHEMBL90039 | diphenylmethanone) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain by spectrofluorimetry | Eur J Med Chem 44: 1230-9 (2009) Article DOI: 10.1016/j.ejmech.2008.09.018 BindingDB Entry DOI: 10.7270/Q26974HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||