

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581204 (CHEMBL5076637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581204 (CHEMBL5076637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581191 (CHEMBL5070876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581203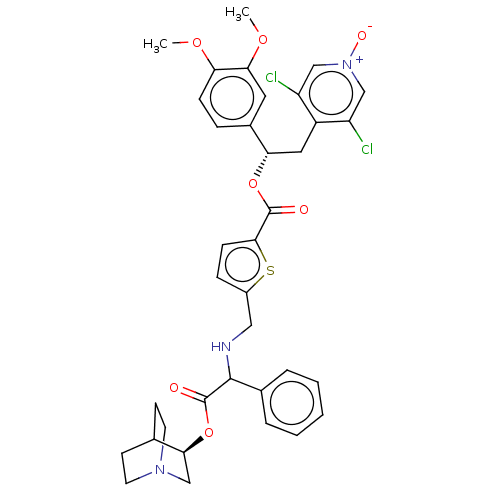 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581209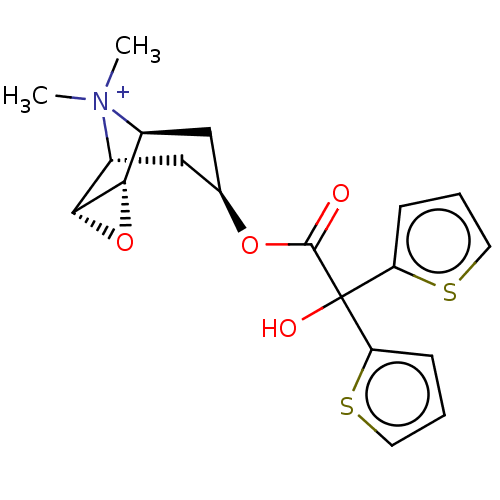 (CHEMBL4650755) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581192 (CHEMBL5091461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581203 (CHEMBL5074599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581185 (CHEMBL5076558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50581209 (CHEMBL4650755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581187 (CHEMBL5077161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581189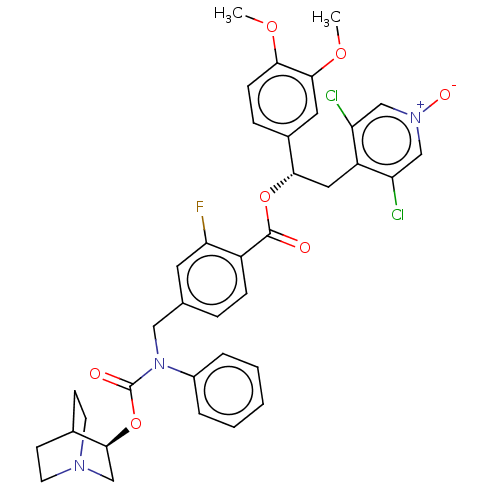 (CHEMBL5075132) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50581203 (CHEMBL5074599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581202 (CHEMBL5090464) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581193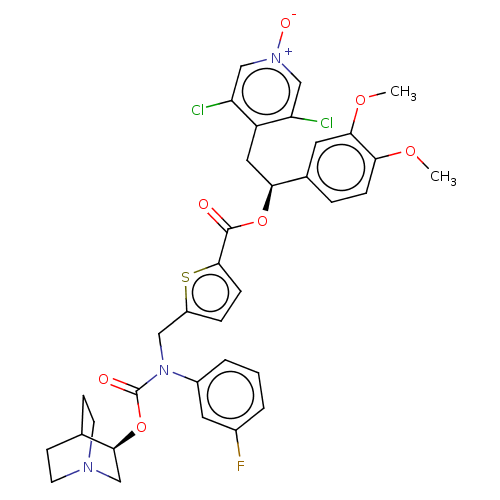 (CHEMBL5084383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581190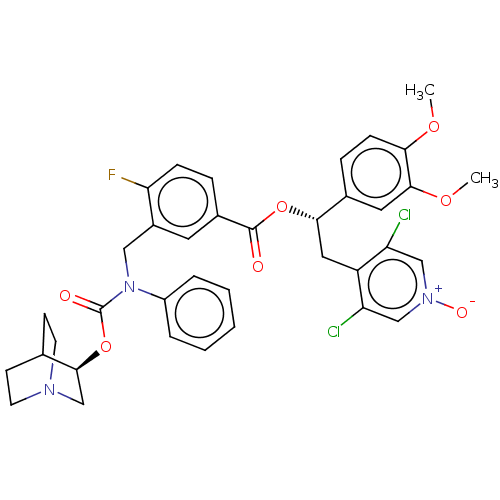 (CHEMBL5076266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325610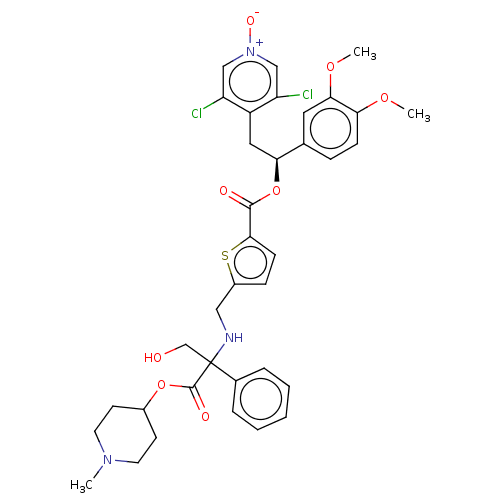 (US9636336, Example 105c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581199 (CHEMBL5090179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325606 (US9636336, Example 87c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581188 (CHEMBL5076680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581186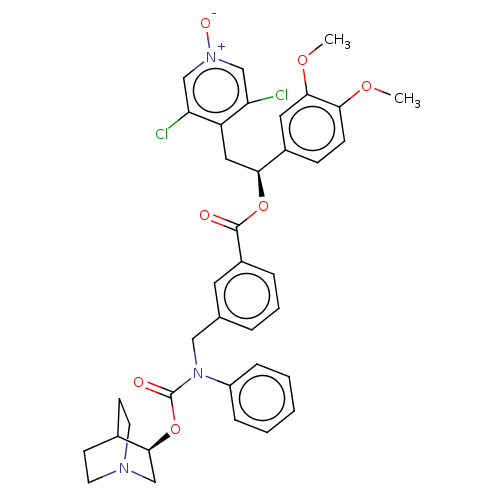 (CHEMBL5088742) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581201 (CHEMBL5085717) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581200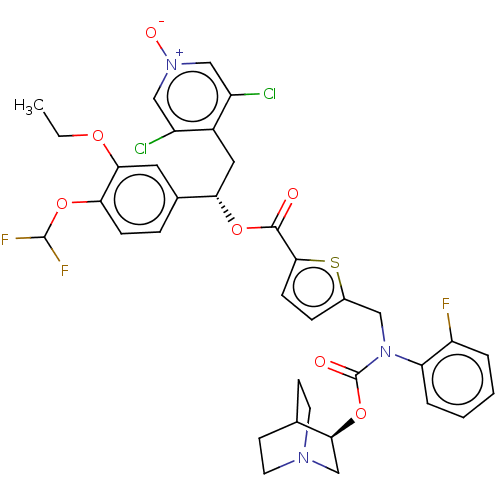 (CHEMBL5084829) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581198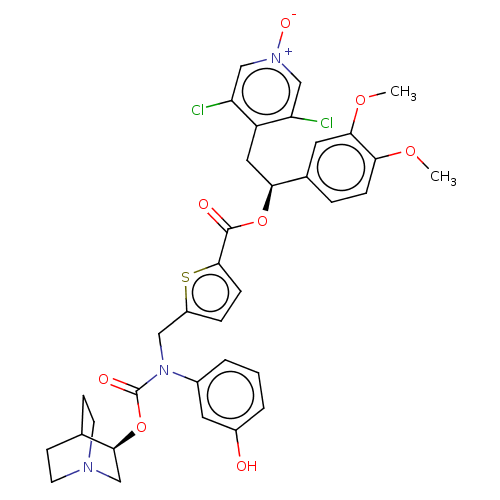 (CHEMBL5086769) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581185 (CHEMBL5076558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581185 (CHEMBL5076558) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581183 (CHEMBL5087564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581206 (CHEMBL5076886) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581195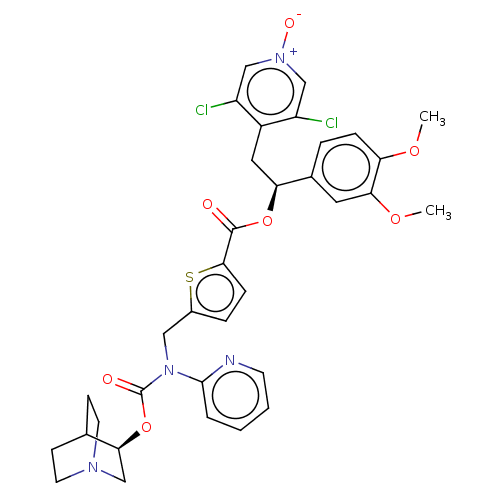 (CHEMBL5085166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581206 (CHEMBL5076886) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325608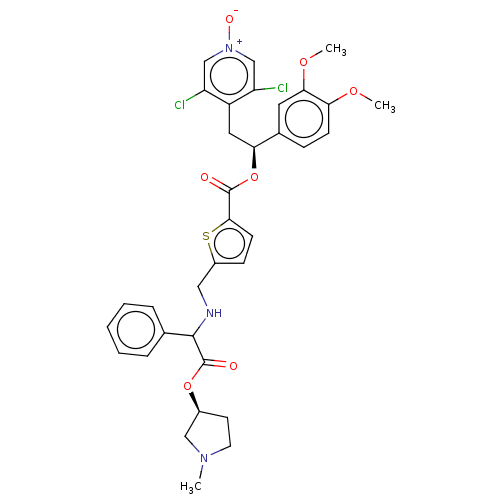 (US9636336, Example 78c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325607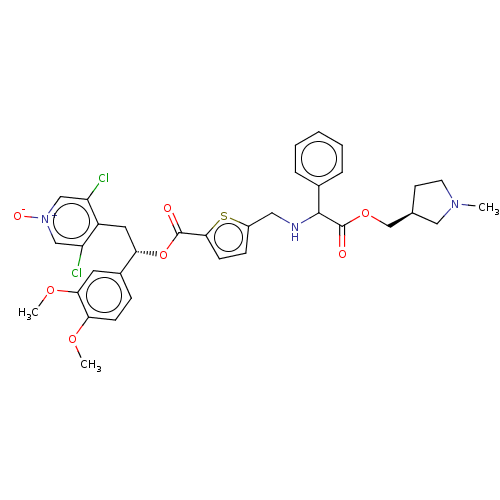 (US9636336, Example 18 | US9636336, Example 77 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581184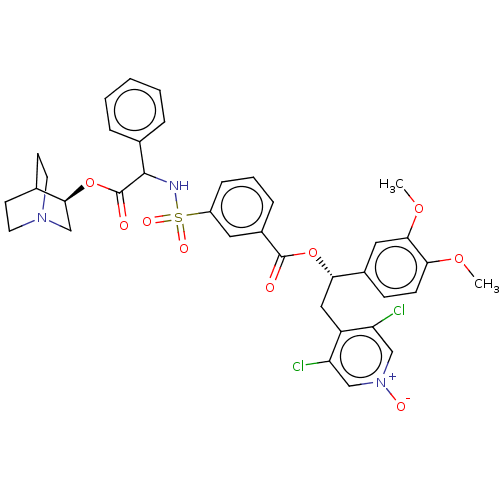 (CHEMBL5094110) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581181 (CHEMBL5086895) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581205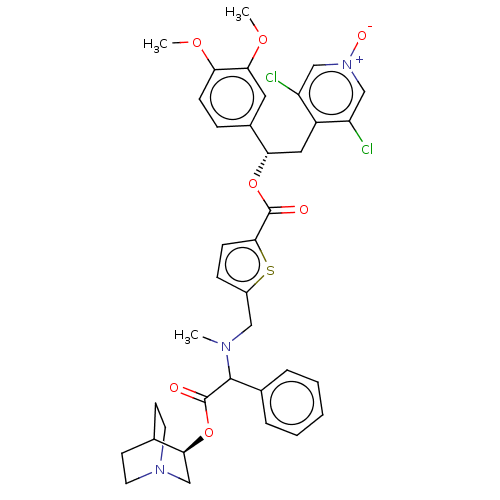 (CHEMBL5077424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581205 (CHEMBL5077424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50208047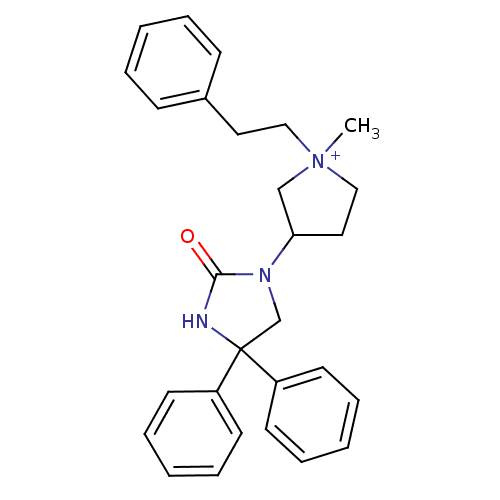 (1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325605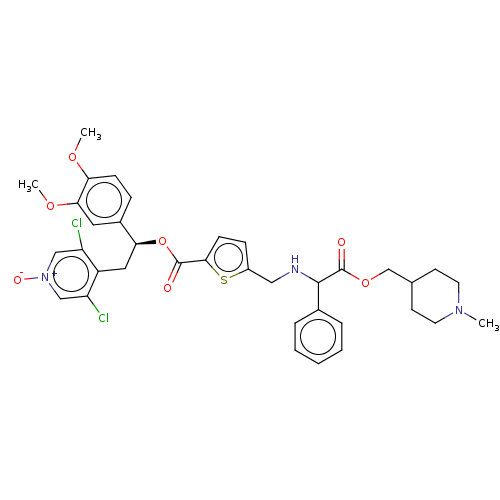 (US9636336, Example 23 | US9636336, Example 86 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50208057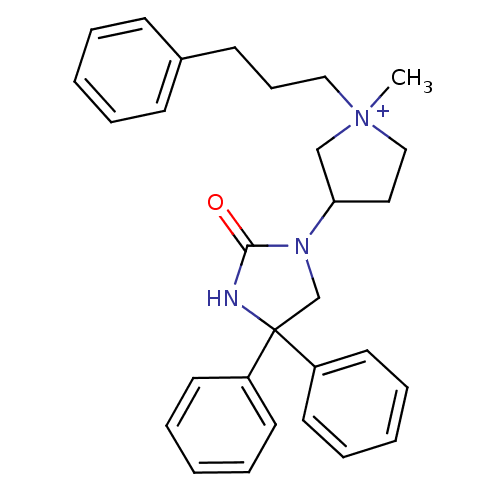 (1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50208057 (1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50208043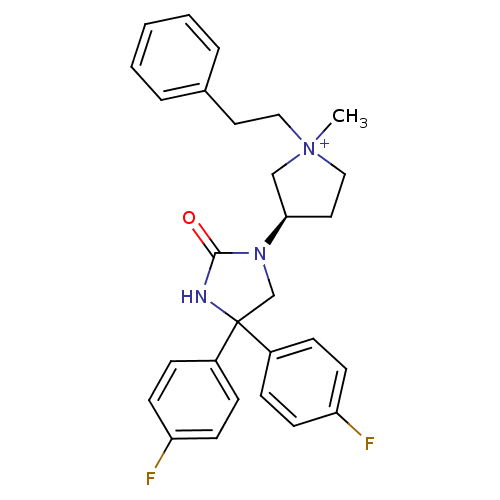 (1-methyl-3-(R)-[4,4-Bis-(4-fluoro-phenyl)-2-oxo-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581179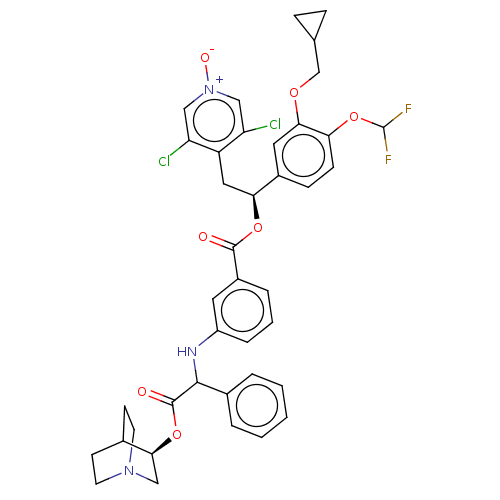 (CHEMBL5078680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50581210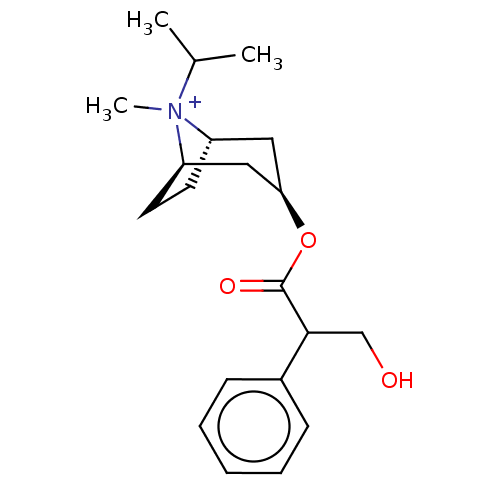 (IPRATROPIUM | Ipratropium | Ipratropium cation | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581210 (IPRATROPIUM | Ipratropium | Ipratropium cation | I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581196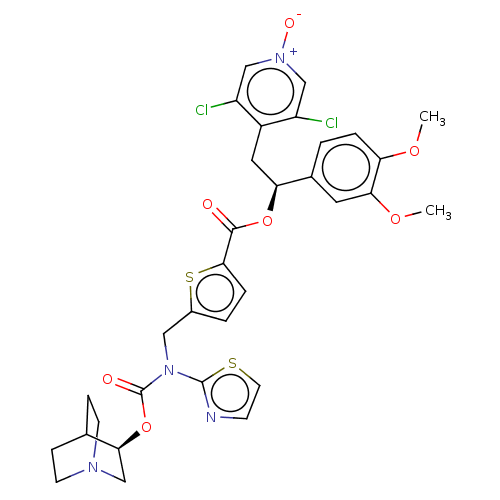 (CHEMBL5086272) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325609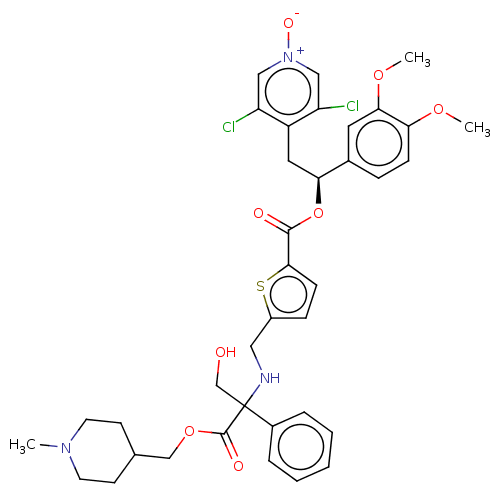 (US9636336, Example 105 | US9636336, Example 106 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50207996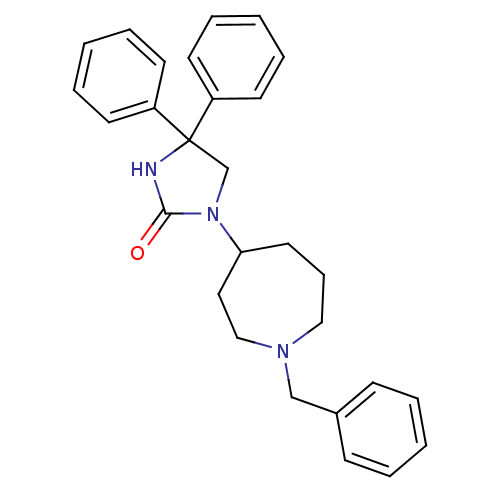 (3-(1-benzyl-azepin-4-yl)-5,5-diphenyl-imidazolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHO K1 cells | J Med Chem 50: 1571-83 (2007) Article DOI: 10.1021/jm061159a BindingDB Entry DOI: 10.7270/Q2QV3M61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50208044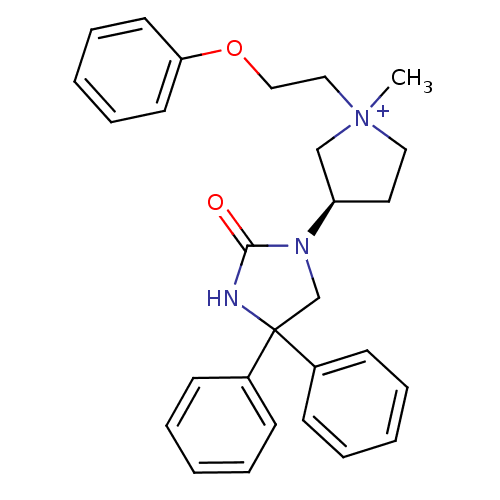 (1-methyl-3-(R)-(2-oxo-4,4-diphenyl-imidazolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581182 (CHEMBL5080391) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50581180 (CHEMBL5081214) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00204 BindingDB Entry DOI: 10.7270/Q2DN48WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50208035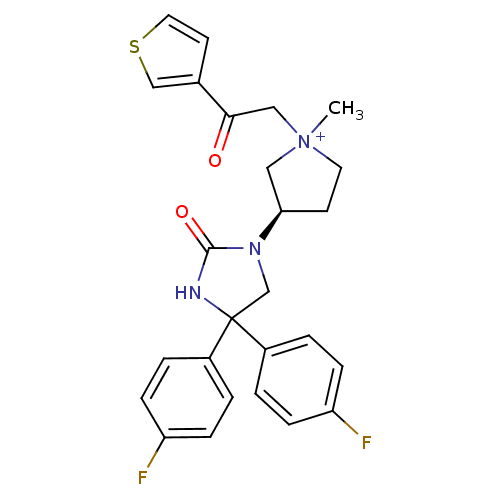 (1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25 Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells | J Med Chem 50: 1693-7 (2007) Article DOI: 10.1021/jm061160+ BindingDB Entry DOI: 10.7270/Q2M32VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 720 total ) | Next | Last >> |