

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286545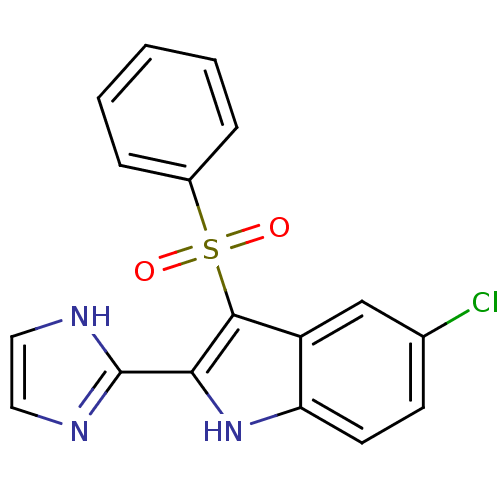 (3-Benzenesulfonyl-5-chloro-2-(1H-imidazol-2-yl)-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50230553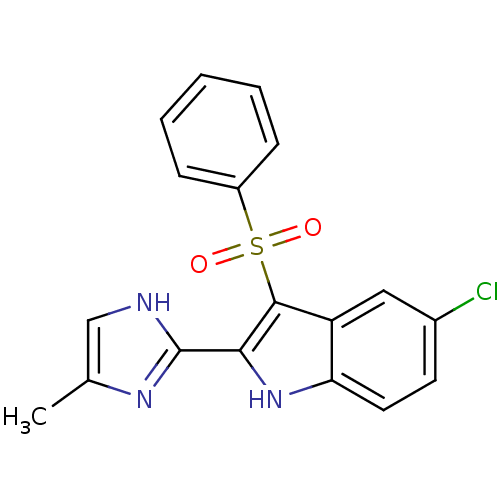 (3-Benzenesulfonyl-5-chloro-2-(5-methyl-1H-imidazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286549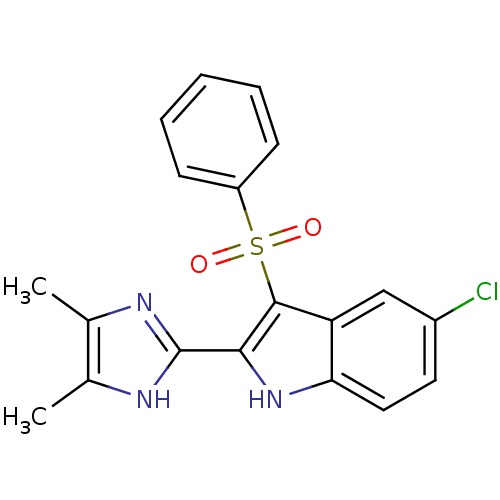 (3-Benzenesulfonyl-5-chloro-2-(4,5-dimethyl-1H-imid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286550 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-1H-imida...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286551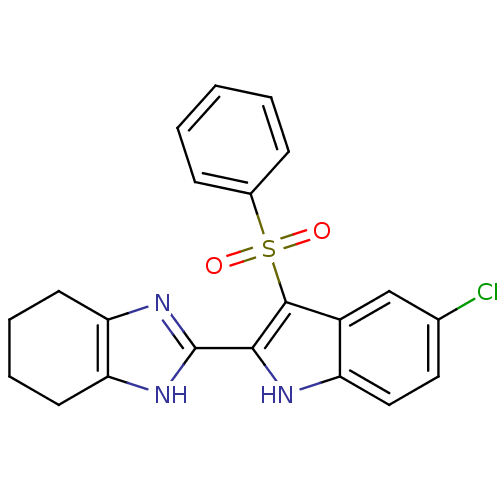 (2-(3-Benzenesulfonyl-5-chloro-1H-indol-2-yl)-4,5,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50230553 (3-Benzenesulfonyl-5-chloro-2-(5-methyl-1H-imidazol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286543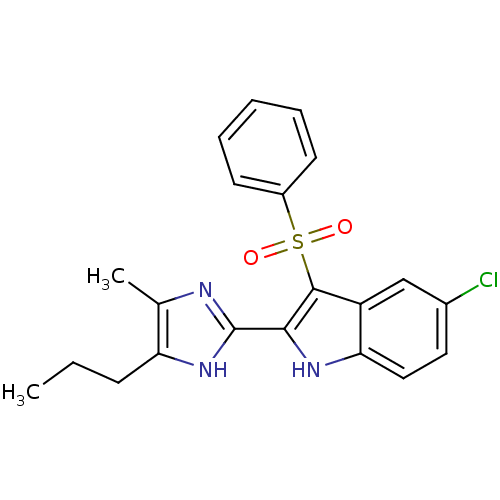 (3-Benzenesulfonyl-5-chloro-2-(4-methyl-5-propyl-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286548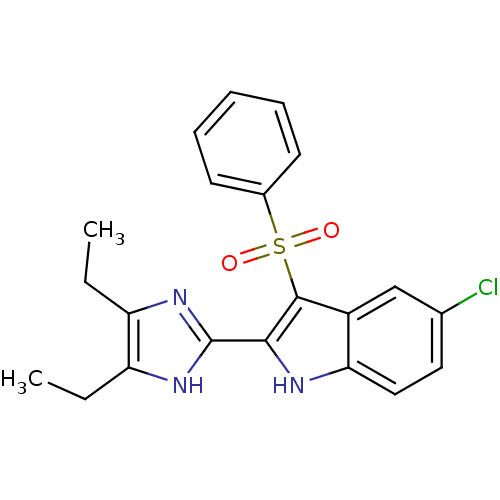 (3-Benzenesulfonyl-5-chloro-2-(4,5-diethyl-1H-imida...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286542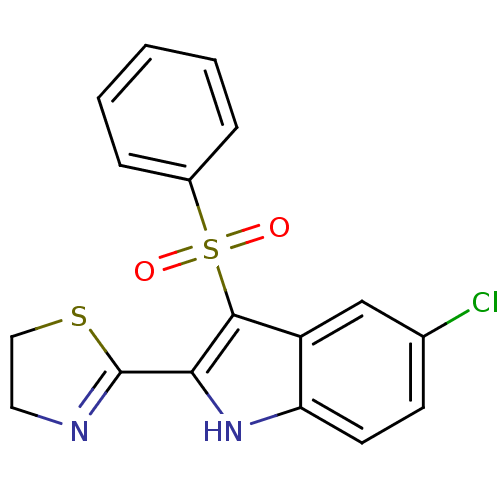 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-thiazol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286546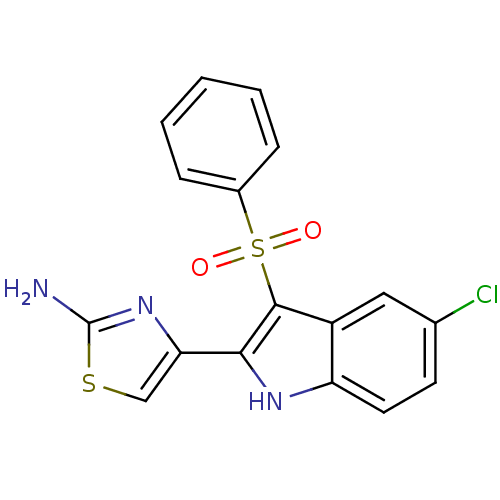 (4-(3-Benzenesulfonyl-5-chloro-1H-indol-2-yl)-4H-1l...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286549 (3-Benzenesulfonyl-5-chloro-2-(4,5-dimethyl-1H-imid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286545 (3-Benzenesulfonyl-5-chloro-2-(1H-imidazol-2-yl)-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286552 ((3aS,7aS)-2-(3-Benzenesulfonyl-5-chloro-1H-indol-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286544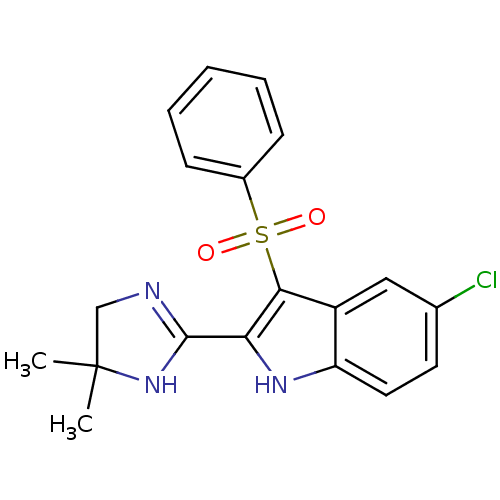 (3-Benzenesulfonyl-5-chloro-2-(5,5-dimethyl-4,5-dih...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286543 (3-Benzenesulfonyl-5-chloro-2-(4-methyl-5-propyl-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286546 (4-(3-Benzenesulfonyl-5-chloro-1H-indol-2-yl)-4H-1l...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286550 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-1H-imida...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286545 (3-Benzenesulfonyl-5-chloro-2-(1H-imidazol-2-yl)-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N/Y181C double mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286542 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-thiazol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286547 (3-Benzenesulfonyl-5-chloro-2-(1,4,5,6-tetrahydro-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286553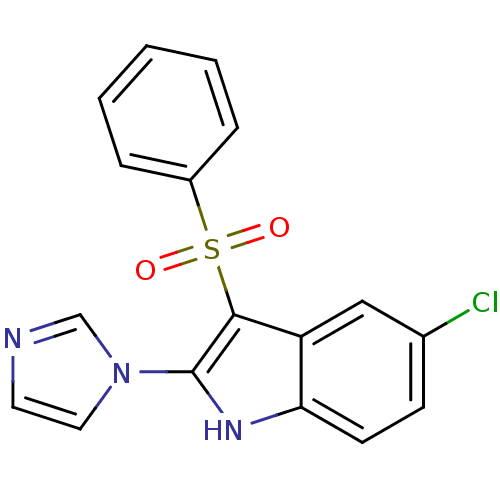 (3-Benzenesulfonyl-5-chloro-2-imidazol-1-yl-1H-indo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primer | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286549 (3-Benzenesulfonyl-5-chloro-2-(4,5-dimethyl-1H-imid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N/Y181C double mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286545 (3-Benzenesulfonyl-5-chloro-2-(1H-imidazol-2-yl)-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286542 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-thiazol-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286549 (3-Benzenesulfonyl-5-chloro-2-(4,5-dimethyl-1H-imid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286550 (3-Benzenesulfonyl-5-chloro-2-(4,5-dihydro-1H-imida...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286543 (3-Benzenesulfonyl-5-chloro-2-(4-methyl-5-propyl-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-2 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50230553 (3-Benzenesulfonyl-5-chloro-2-(5-methyl-1H-imidazol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50286546 (4-(3-Benzenesulfonyl-5-chloro-1H-indol-2-yl)-4H-1l...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against K103N mutant of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 491-496 (1995) Article DOI: 10.1016/0960-894X(95)00059-3 BindingDB Entry DOI: 10.7270/Q28052KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||