 Found 468 hits with Last Name = 'amici' and Initial = 'r'
Found 468 hits with Last Name = 'amici' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Homo sapiens (Human)) | BDBM50141381

(CHEMBL3758184)Show SMILES ONC(=O)\C=C\c1ccc2OC3(CCCN(CCc4ccccc4)C3)CC(=O)c2c1 Show InChI InChI=1S/C24H26N2O4/c27-21-16-24(12-4-13-26(17-24)14-11-18-5-2-1-3-6-18)30-22-9-7-19(15-20(21)22)8-10-23(28)25-29/h1-3,5-10,15,29H,4,11-14,16-17H2,(H,25,28)/b10-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cells nuclear extract using Fluor de lys as substrate after 15 mins by fluorometric analysis |
Eur J Med Chem 108: 53-67 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.010
BindingDB Entry DOI: 10.7270/Q2N87CN0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27391
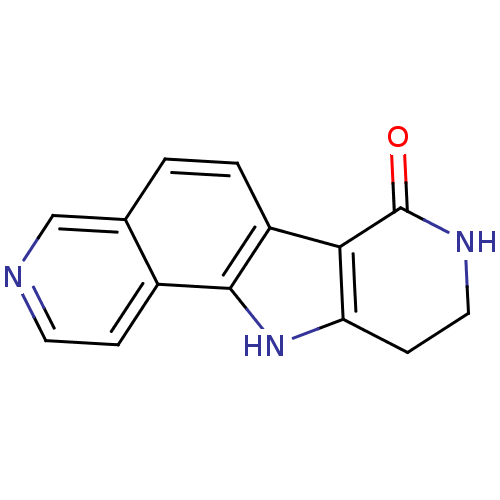
(5,13,17-triazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]h...)Show InChI InChI=1S/C14H11N3O/c18-14-12-10-2-1-8-7-15-5-3-9(8)13(10)17-11(12)4-6-16-14/h1-3,5,7,17H,4,6H2,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27413
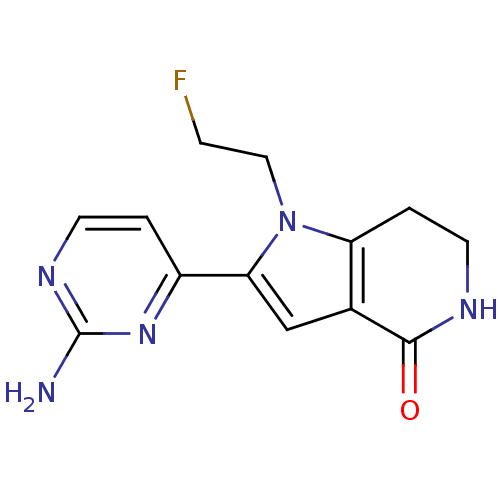
(2-(2-aminopyrimidin-4-yl)-1-(2-fluoroethyl)-1H,4H,...)Show InChI InChI=1S/C13H14FN5O/c14-3-6-19-10-2-5-16-12(20)8(10)7-11(19)9-1-4-17-13(15)18-9/h1,4,7H,2-3,5-6H2,(H,16,20)(H2,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7163

(3-Phenylacetamidoaminopyrazole deriv. 40 | CS10 | ...)Show InChI InChI=1S/C18H17N3OS/c22-18(19-17-11-15(20-21-17)13-7-8-13)10-12-3-5-14(6-4-12)16-2-1-9-23-16/h1-6,9,11,13H,7-8,10H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27412

(2-(2-aminopyrimidin-4-yl)-1-(cyclopropylmethyl)-1H...)Show InChI InChI=1S/C15H17N5O/c16-15-18-5-3-11(19-15)13-7-10-12(4-6-17-14(10)21)20(13)8-9-1-2-9/h3,5,7,9H,1-2,4,6,8H2,(H,17,21)(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27390

(2-(2-amino-5-bromopyrimidin-4-yl)-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C11H10BrN5O/c12-6-4-15-11(13)17-9(6)8-3-5-7(16-8)1-2-14-10(5)18/h3-4,16H,1-2H2,(H,14,18)(H2,13,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7162

(3-Phenylacetamidoaminopyrazole deriv. 39 | 4 -{2-[...)Show SMILES NC(=O)c1ccc(cc1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H20N4O2/c22-21(27)17-9-5-15(6-10-17)14-3-1-13(2-4-14)11-20(26)23-19-12-18(24-25-19)16-7-8-16/h1-6,9-10,12,16H,7-8,11H2,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7159
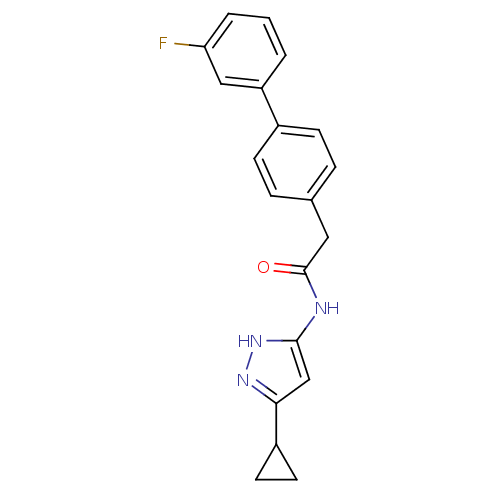
(3-Phenylacetamidoaminopyrazole deriv. 36 | N-(5-Cy...)Show SMILES Fc1cccc(c1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C20H18FN3O/c21-17-3-1-2-16(11-17)14-6-4-13(5-7-14)10-20(25)22-19-12-18(23-24-19)15-8-9-15/h1-7,11-12,15H,8-10H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27406
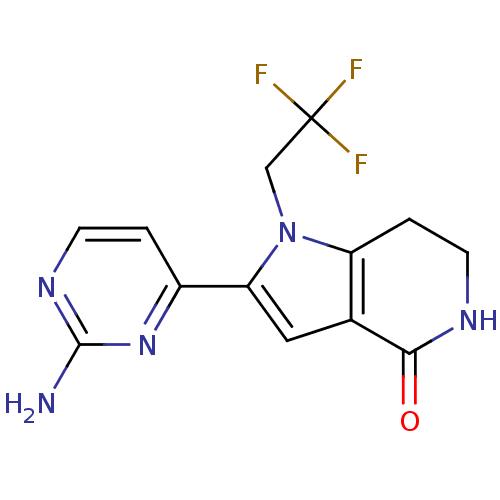
(2-(2-aminopyrimidin-4-yl)-1-(2,2,2-trifluoroethyl)...)Show InChI InChI=1S/C13H12F3N5O/c14-13(15,16)6-21-9-2-4-18-11(22)7(9)5-10(21)8-1-3-19-12(17)20-8/h1,3,5H,2,4,6H2,(H,18,22)(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50378657
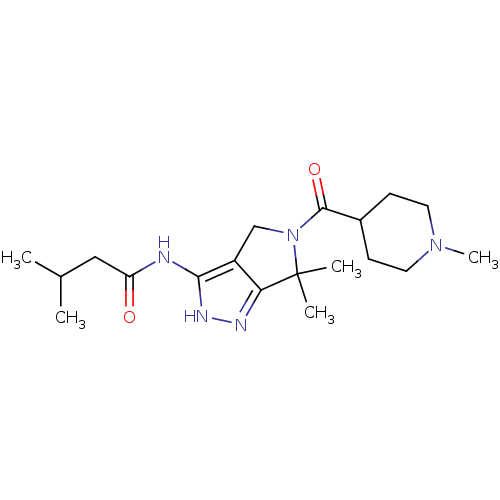
(CHEMBL1230607)Show SMILES CC(C)CC(=O)Nc1[nH]nc2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C19H31N5O2/c1-12(2)10-15(25)20-17-14-11-24(19(3,4)16(14)21-22-17)18(26)13-6-8-23(5)9-7-13/h12-13H,6-11H2,1-5H3,(H2,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027427

(CHEMBL1744453)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3ccsc3)n[nH]c2C1(C)C Show InChI InChI=1S/C19H25N5O2S/c1-19(2)15-14(10-24(19)18(26)12-4-7-23(3)8-5-12)16(22-21-15)20-17(25)13-6-9-27-11-13/h6,9,11-12H,4-5,7-8,10H2,1-3H3,(H2,20,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27421
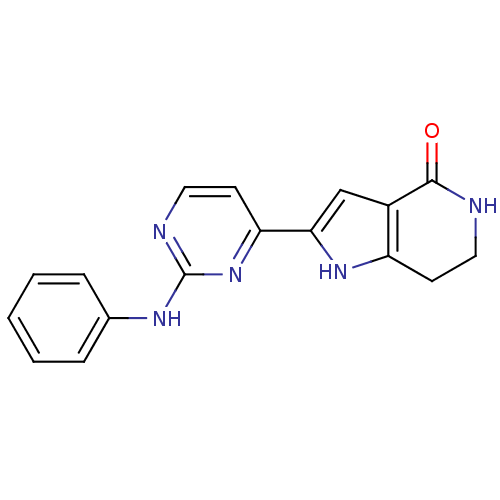
(2-[2-(phenylamino)pyrimidin-4-yl]-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C17H15N5O/c23-16-12-10-15(21-13(12)6-8-18-16)14-7-9-19-17(22-14)20-11-4-2-1-3-5-11/h1-5,7,9-10,21H,6,8H2,(H,18,23)(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27351
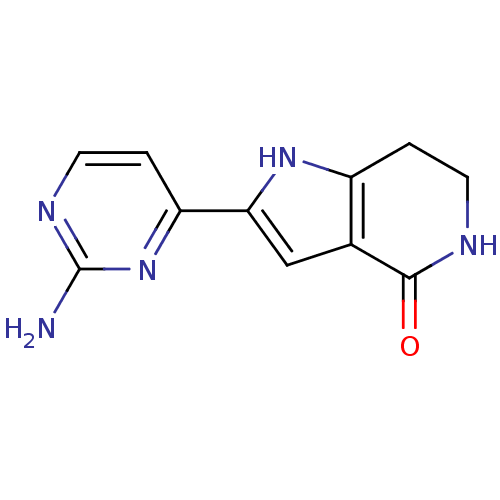
(2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...)Show InChI InChI=1S/C11H11N5O/c12-11-14-4-2-8(16-11)9-5-6-7(15-9)1-3-13-10(6)17/h2,4-5,15H,1,3H2,(H,13,17)(H2,12,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27404
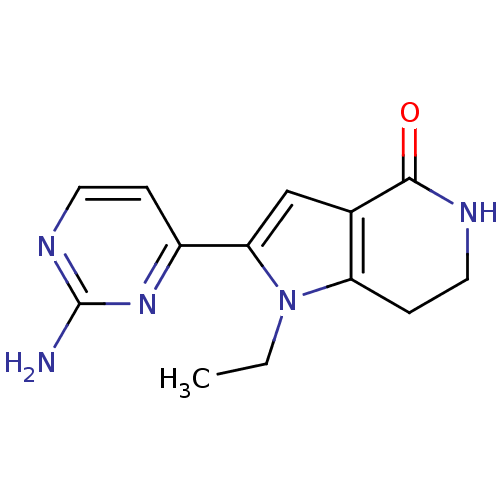
(2-(2-aminopyrimidin-4-yl)-1-ethyl-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C13H15N5O/c1-2-18-10-4-6-15-12(19)8(10)7-11(18)9-3-5-16-13(14)17-9/h3,5,7H,2,4,6H2,1H3,(H,15,19)(H2,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50378657

(CHEMBL1230607)Show SMILES CC(C)CC(=O)Nc1[nH]nc2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C19H31N5O2/c1-12(2)10-15(25)20-17-14-11-24(19(3,4)16(14)21-22-17)18(26)13-6-8-23(5)9-7-13/h12-13H,6-11H2,1-5H3,(H2,20,21,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50378657

(CHEMBL1230607)Show SMILES CC(C)CC(=O)Nc1[nH]nc2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C19H31N5O2/c1-12(2)10-15(25)20-17-14-11-24(19(3,4)16(14)21-22-17)18(26)13-6-8-23(5)9-7-13/h12-13H,6-11H2,1-5H3,(H2,20,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027423

(CHEMBL1744456)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)C3CCC3)n[nH]c2C1(C)C Show InChI InChI=1S/C19H29N5O2/c1-19(2)15-14(16(22-21-15)20-17(25)12-5-4-6-12)11-24(19)18(26)13-7-9-23(3)10-8-13/h12-13H,4-11H2,1-3H3,(H2,20,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27382
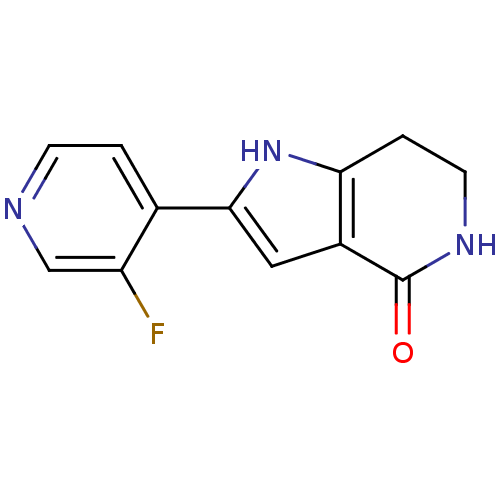
(2-(3-fluoropyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...)Show InChI InChI=1S/C12H10FN3O/c13-9-6-14-3-1-7(9)11-5-8-10(16-11)2-4-15-12(8)17/h1,3,5-6,16H,2,4H2,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7160
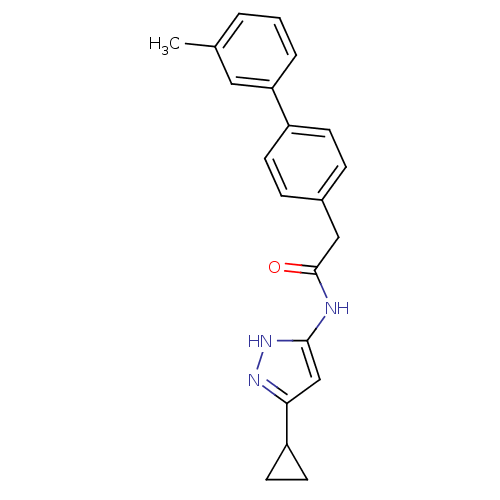
(3-Phenylacetamidoaminopyrazole deriv. 37 | N-(5-Cy...)Show SMILES Cc1cccc(c1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H21N3O/c1-14-3-2-4-18(11-14)16-7-5-15(6-8-16)12-21(25)22-20-13-19(23-24-20)17-9-10-17/h2-8,11,13,17H,9-10,12H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027430

(CHEMBL1744452)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3cccs3)n[nH]c2C1(C)C Show InChI InChI=1S/C19H25N5O2S/c1-19(2)15-13(11-24(19)18(26)12-6-8-23(3)9-7-12)16(22-21-15)20-17(25)14-5-4-10-27-14/h4-5,10,12H,6-9,11H2,1-3H3,(H2,20,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123468
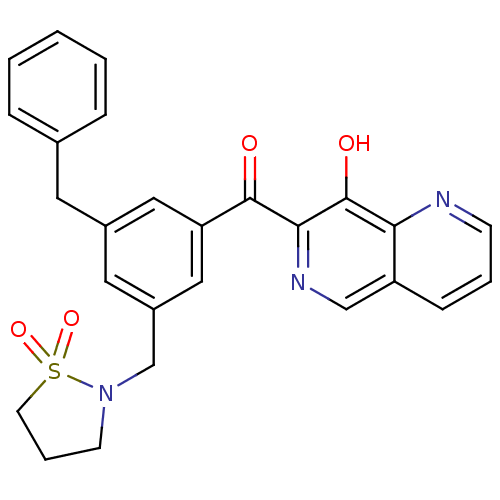
(CHEMBL32865 | [3-Benzyl-5-(1,1-dioxo-1lambda 6 -is...)Show SMILES Oc1c(ncc2cccnc12)C(=O)c1cc(CN2CCCS2(=O)=O)cc(Cc2ccccc2)c1 Show InChI InChI=1S/C26H23N3O4S/c30-25(24-26(31)23-21(16-28-24)8-4-9-27-23)22-14-19(12-18-6-2-1-3-7-18)13-20(15-22)17-29-10-5-11-34(29,32)33/h1-4,6-9,13-16,31H,5,10-12,17H2 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Cenci BolognettiUniversit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase strand transfer activity by high throughput electrochemiluminescent assay in presence of magnesium |
J Med Chem 51: 4744-50 (2008)
Article DOI: 10.1021/jm8001422
BindingDB Entry DOI: 10.7270/Q2GM8B3P |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027392
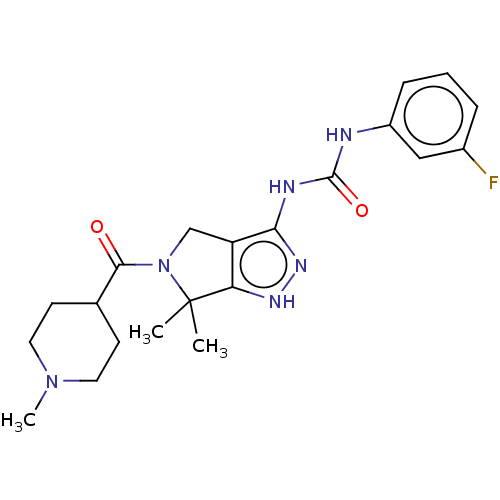
(CHEMBL596978)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)Nc3cccc(F)c3)n[nH]c2C1(C)C Show InChI InChI=1S/C21H27FN6O2/c1-21(2)17-16(12-28(21)19(29)13-7-9-27(3)10-8-13)18(26-25-17)24-20(30)23-15-6-4-5-14(22)11-15/h4-6,11,13H,7-10,12H2,1-3H3,(H3,23,24,25,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50378657

(CHEMBL1230607)Show SMILES CC(C)CC(=O)Nc1[nH]nc2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C19H31N5O2/c1-12(2)10-15(25)20-17-14-11-24(19(3,4)16(14)21-22-17)18(26)13-6-8-23(5)9-7-13/h12-13H,6-11H2,1-5H3,(H2,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK7/cyclin H by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27344
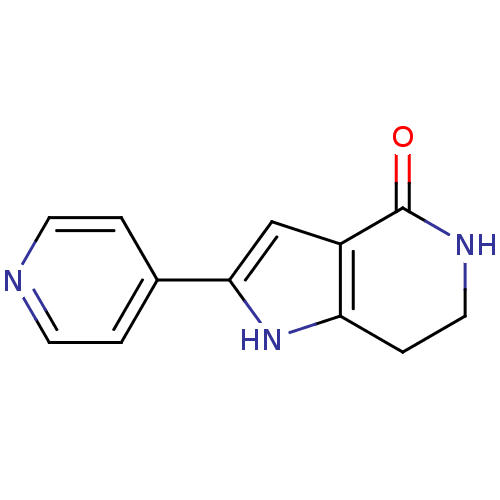
(2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...)Show InChI InChI=1S/C12H11N3O/c16-12-9-7-11(8-1-4-13-5-2-8)15-10(9)3-6-14-12/h1-2,4-5,7,15H,3,6H2,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027396

(CHEMBL603243)Show SMILES CCCNC(=O)Nc1n[nH]c2c1CN(C(=O)C1CCN(C)CC1)C2(C)C Show InChI InChI=1S/C18H30N6O2/c1-5-8-19-17(26)20-15-13-11-24(18(2,3)14(13)21-22-15)16(25)12-6-9-23(4)10-7-12/h12H,5-11H2,1-4H3,(H3,19,20,21,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7161
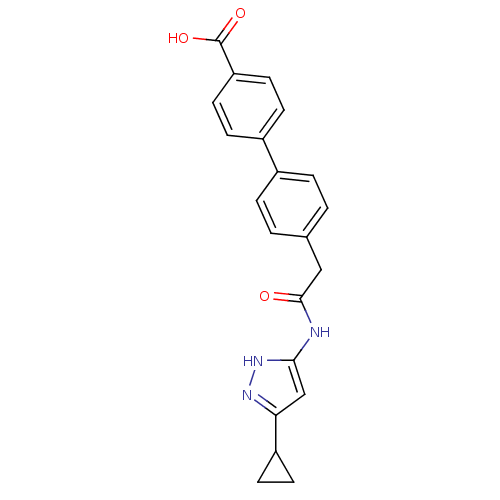
(3-Phenylacetamidoaminopyrazole deriv. 38 | 4 -{2-[...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H19N3O3/c25-20(22-19-12-18(23-24-19)16-7-8-16)11-13-1-3-14(4-2-13)15-5-9-17(10-6-15)21(26)27/h1-6,9-10,12,16H,7-8,11H2,(H,26,27)(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481203
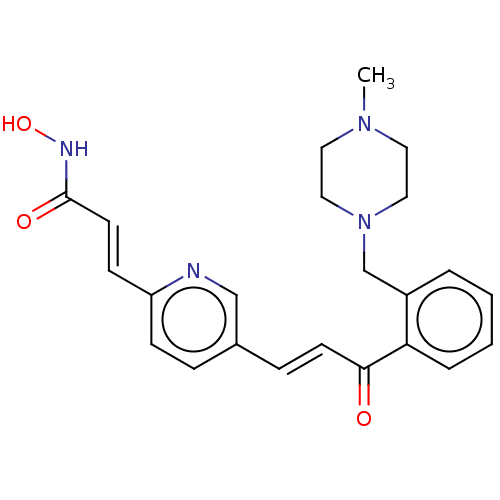
(CHEMBL569327)Show SMILES CN1CCN(Cc2ccccc2C(=O)\C=C\c2ccc(\C=C\C(=O)NO)nc2)CC1 Show InChI InChI=1S/C23H26N4O3/c1-26-12-14-27(15-13-26)17-19-4-2-3-5-21(19)22(28)10-7-18-6-8-20(24-16-18)9-11-23(29)25-30/h2-11,16,30H,12-15,17H2,1H3,(H,25,29)/b10-7+,11-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Congenia s.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC extracted from human HeLa cells |
J Med Chem 53: 822-39 (2010)
Article DOI: 10.1021/jm901502p
BindingDB Entry DOI: 10.7270/Q2CR5X5X |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50184349
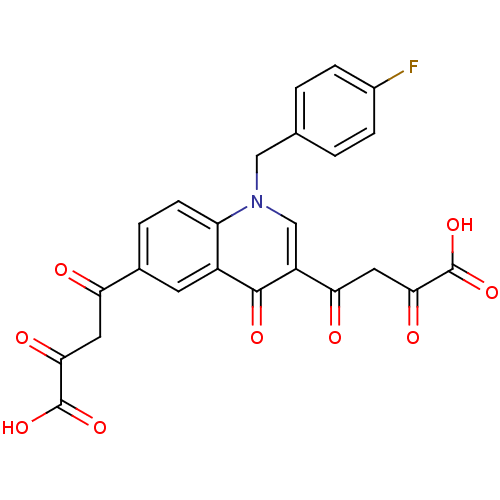
((Z)-4-[6-((Z)-3-carboxy-3-hydroxy-acryloyl)-1-(4-f...)Show SMILES OC(=O)C(=O)CC(=O)c1ccc2n(Cc3ccc(F)cc3)cc(C(=O)CC(=O)C(O)=O)c(=O)c2c1 Show InChI InChI=1S/C24H16FNO9/c25-14-4-1-12(2-5-14)10-26-11-16(19(28)9-21(30)24(34)35)22(31)15-7-13(3-6-17(15)26)18(27)8-20(29)23(32)33/h1-7,11H,8-10H2,(H,32,33)(H,34,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in presence of MnCl2 |
J Med Chem 49: 1939-45 (2006)
Article DOI: 10.1021/jm0511583
BindingDB Entry DOI: 10.7270/Q2GT5MRF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27407

(2-(2-aminopyrimidin-4-yl)-1-propyl-1H,4H,5H,6H,7H-...)Show InChI InChI=1S/C14H17N5O/c1-2-7-19-11-4-6-16-13(20)9(11)8-12(19)10-3-5-17-14(15)18-10/h3,5,8H,2,4,6-7H2,1H3,(H,16,20)(H2,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27411
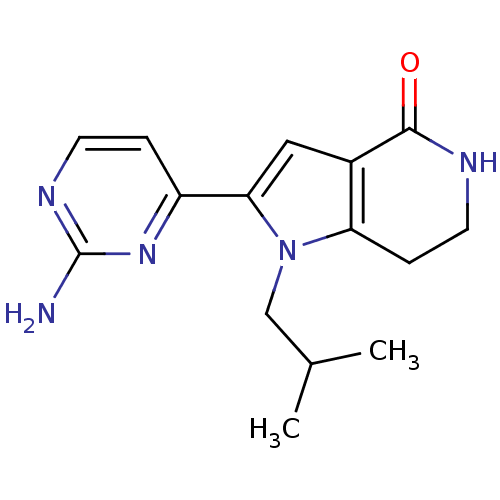
(2-(2-aminopyrimidin-4-yl)-1-(2-methylpropyl)-1H,4H...)Show InChI InChI=1S/C15H19N5O/c1-9(2)8-20-12-4-6-17-14(21)10(12)7-13(20)11-3-5-18-15(16)19-11/h3,5,7,9H,4,6,8H2,1-2H3,(H,17,21)(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7121
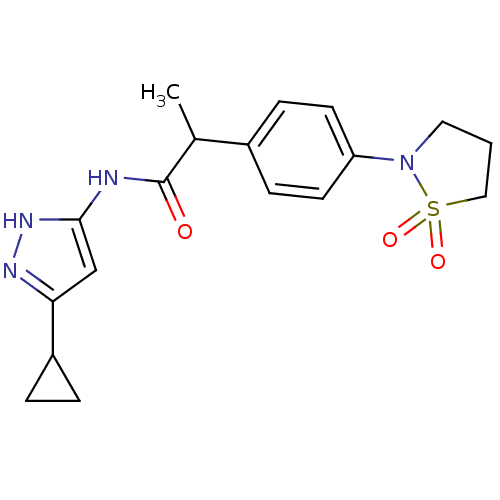
(3-Aminopyrazole deriv. 36 | N-(5-Cyclopropyl-1H-py...)Show SMILES CC(C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)N1CCCS1(=O)=O Show InChI InChI=1S/C18H22N4O3S/c1-12(18(23)19-17-11-16(20-21-17)14-3-4-14)13-5-7-15(8-6-13)22-9-2-10-26(22,24)25/h5-8,11-12,14H,2-4,9-10H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27384
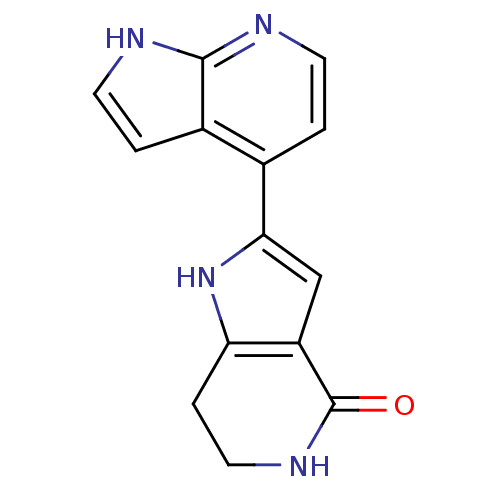
(2-{1H-pyrrolo[2,3-b]pyridin-4-yl}-1H,4H,5H,6H,7H-p...)Show InChI InChI=1S/C14H12N4O/c19-14-10-7-12(18-11(10)3-6-17-14)8-1-4-15-13-9(8)2-5-16-13/h1-2,4-5,7,18H,3,6H2,(H,15,16)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM27409
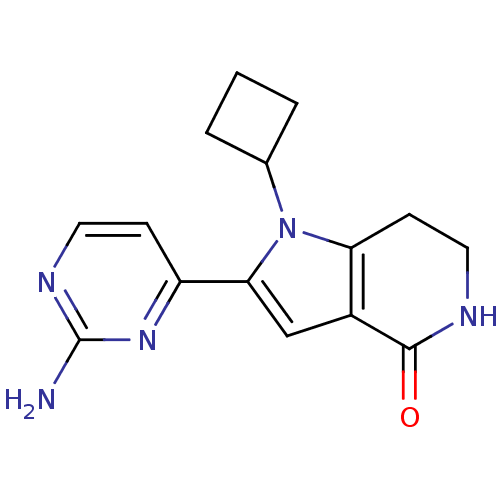
(2-(2-aminopyrimidin-4-yl)-1-cyclobutyl-1H,4H,5H,6H...)Show InChI InChI=1S/C15H17N5O/c16-15-18-6-4-11(19-15)13-8-10-12(5-7-17-14(10)21)20(13)9-2-1-3-9/h4,6,8-9H,1-3,5,7H2,(H,17,21)(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
| Assay Description
The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... |
J Med Chem 51: 487-501 (2008)
Article DOI: 10.1021/jm700956r
BindingDB Entry DOI: 10.7270/Q247485B |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027421
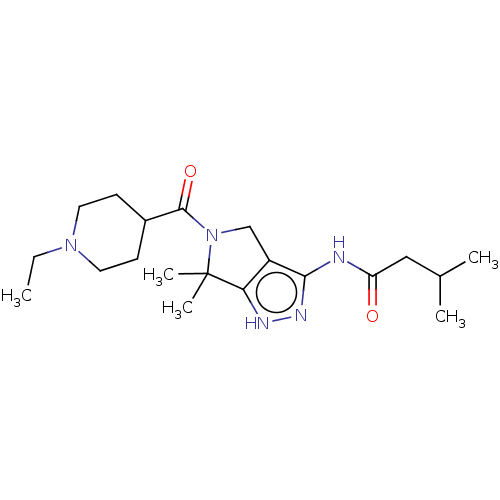
(CHEMBL1744457)Show SMILES CCN1CCC(CC1)C(=O)N1Cc2c(NC(=O)CC(C)C)n[nH]c2C1(C)C Show InChI InChI=1S/C20H33N5O2/c1-6-24-9-7-14(8-10-24)19(27)25-12-15-17(20(25,4)5)22-23-18(15)21-16(26)11-13(2)3/h13-14H,6-12H2,1-5H3,(H2,21,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027404
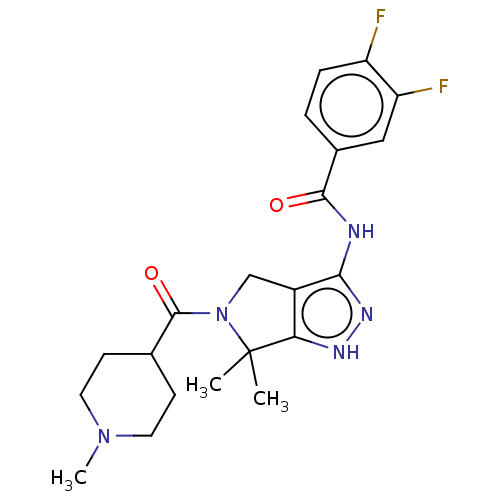
(CHEMBL603478)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3ccc(F)c(F)c3)n[nH]c2C1(C)C Show InChI InChI=1S/C21H25F2N5O2/c1-21(2)17-14(11-28(21)20(30)12-6-8-27(3)9-7-12)18(26-25-17)24-19(29)13-4-5-15(22)16(23)10-13/h4-5,10,12H,6-9,11H2,1-3H3,(H2,24,25,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027416
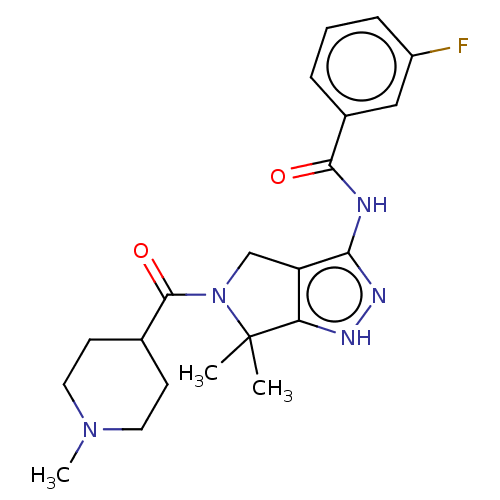
(CHEMBL599249)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3cccc(F)c3)n[nH]c2C1(C)C Show InChI InChI=1S/C21H26FN5O2/c1-21(2)17-16(12-27(21)20(29)13-7-9-26(3)10-8-13)18(25-24-17)23-19(28)14-5-4-6-15(22)11-14/h4-6,11,13H,7-10,12H2,1-3H3,(H2,23,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481193

(CHEMBL569731)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)\C=C\c1cccc(\C=C\C(=O)NO)n1 Show InChI InChI=1S/C22H24N4O3/c1-25-13-15-26(16-14-25)20-9-5-17(6-10-20)21(27)11-7-18-3-2-4-19(23-18)8-12-22(28)24-29/h2-12,29H,13-16H2,1H3,(H,24,28)/b11-7+,12-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Congenia s.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC extracted from human HeLa cells |
J Med Chem 53: 822-39 (2010)
Article DOI: 10.1021/jm901502p
BindingDB Entry DOI: 10.7270/Q2CR5X5X |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027413
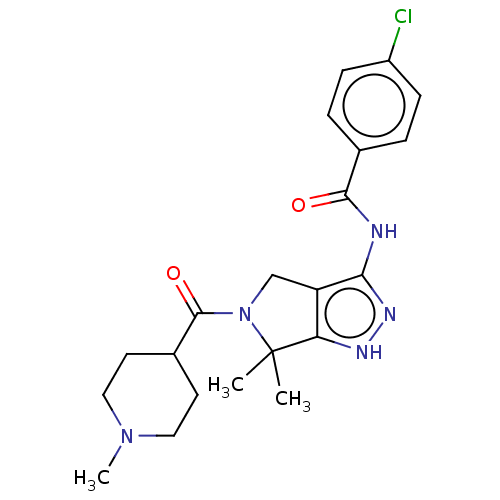
(CHEMBL598221)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3ccc(Cl)cc3)n[nH]c2C1(C)C Show InChI InChI=1S/C21H26ClN5O2/c1-21(2)17-16(12-27(21)20(29)14-8-10-26(3)11-9-14)18(25-24-17)23-19(28)13-4-6-15(22)7-5-13/h4-7,14H,8-12H2,1-3H3,(H2,23,24,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027424

(CHEMBL1744455)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)C3CC3)n[nH]c2C1(C)C Show InChI InChI=1S/C18H27N5O2/c1-18(2)14-13(15(21-20-14)19-16(24)11-4-5-11)10-23(18)17(25)12-6-8-22(3)9-7-12/h11-12H,4-10H2,1-3H3,(H2,19,20,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027411
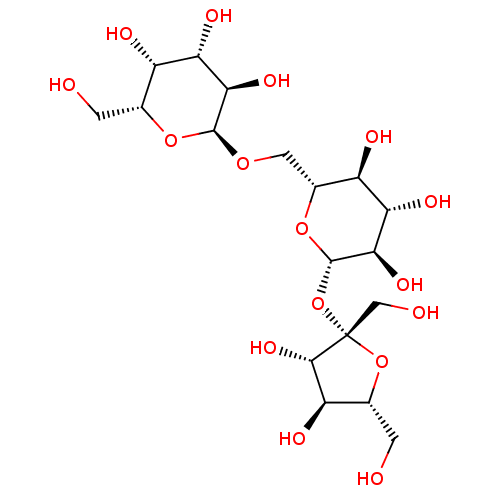
(CHEMBL1744450)Show SMILES OC[C@H]1O[C@@](CO)(O[C@H]2O[C@H](CO[C@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@@H]1O.OC[C@H]1O[C@@](CO)(O[C@@H]2O[C@H](CO[C@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C18H32O16/c19-1-5-8(22)11(25)13(27)16(31-5)30-3-7-9(23)12(26)14(28)17(32-7)34-18(4-21)15(29)10(24)6(2-20)33-18/h5-17,19-29H,1-4H2/t5-,6-,7-,8+,9-,10-,11+,12+,13-,14-,15+,16+,17+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50027403
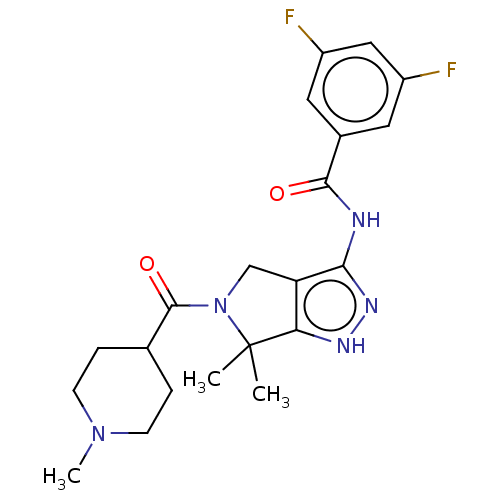
(CHEMBL603887)Show SMILES CN1CCC(CC1)C(=O)N1Cc2c(NC(=O)c3cc(F)cc(F)c3)n[nH]c2C1(C)C Show InChI InChI=1S/C21H25F2N5O2/c1-21(2)17-16(11-28(21)20(30)12-4-6-27(3)7-5-12)18(26-25-17)24-19(29)13-8-14(22)10-15(23)9-13/h8-10,12H,4-7,11H2,1-3H3,(H2,24,25,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay |
Bioorg Med Chem 18: 1844-53 (2010)
Article DOI: 10.1016/j.bmc.2010.01.042
BindingDB Entry DOI: 10.7270/Q21R6RG5 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50184349

((Z)-4-[6-((Z)-3-carboxy-3-hydroxy-acryloyl)-1-(4-f...)Show SMILES OC(=O)C(=O)CC(=O)c1ccc2n(Cc3ccc(F)cc3)cc(C(=O)CC(=O)C(O)=O)c(=O)c2c1 Show InChI InChI=1S/C24H16FNO9/c25-14-4-1-12(2-5-14)10-26-11-16(19(28)9-21(30)24(34)35)22(31)15-7-13(3-6-17(15)26)18(27)8-20(29)23(32)33/h1-7,11H,8-10H2,(H,32,33)(H,34,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in presence of MgCl2 by plate-based electrochemiluminescent assay |
J Med Chem 49: 1939-45 (2006)
Article DOI: 10.1021/jm0511583
BindingDB Entry DOI: 10.7270/Q2GT5MRF |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50479082
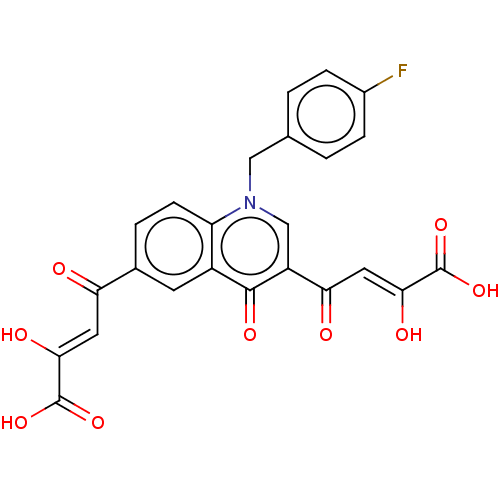
(CHEMBL497501)Show SMILES OC(=O)C(\O)=C\C(=O)c1ccc2n(Cc3ccc(F)cc3)cc(C(=O)\C=C(/O)C(O)=O)c(=O)c2c1 Show InChI InChI=1S/C24H16FNO9/c25-14-4-1-12(2-5-14)10-26-11-16(19(28)9-21(30)24(34)35)22(31)15-7-13(3-6-17(15)26)18(27)8-20(29)23(32)33/h1-9,11,29-30H,10H2,(H,32,33)(H,34,35)/b20-8-,21-9- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Cenci BolognettiUniversit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase strand transfer activity by high throughput electrochemiluminescent assay in presence of magnesium |
J Med Chem 51: 4744-50 (2008)
Article DOI: 10.1021/jm8001422
BindingDB Entry DOI: 10.7270/Q2GM8B3P |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481189

(CHEMBL569546)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)\C=C\c2cccc(\C=C\C(=O)NO)c2)CC1 Show InChI InChI=1S/C24H27N3O3/c1-26-13-15-27(16-14-26)18-21-5-9-22(10-6-21)23(28)11-7-19-3-2-4-20(17-19)8-12-24(29)25-30/h2-12,17,30H,13-16,18H2,1H3,(H,25,29)/b11-7+,12-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Congenia s.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC extracted from human HeLa cells |
J Med Chem 53: 822-39 (2010)
Article DOI: 10.1021/jm901502p
BindingDB Entry DOI: 10.7270/Q2CR5X5X |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50184349

((Z)-4-[6-((Z)-3-carboxy-3-hydroxy-acryloyl)-1-(4-f...)Show SMILES OC(=O)C(=O)CC(=O)c1ccc2n(Cc3ccc(F)cc3)cc(C(=O)CC(=O)C(O)=O)c(=O)c2c1 Show InChI InChI=1S/C24H16FNO9/c25-14-4-1-12(2-5-14)10-26-11-16(19(28)9-21(30)24(34)35)22(31)15-7-13(3-6-17(15)26)18(27)8-20(29)23(32)33/h1-7,11H,8-10H2,(H,32,33)(H,34,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in presence of MgCl2 by gel-based assay |
J Med Chem 49: 1939-45 (2006)
Article DOI: 10.1021/jm0511583
BindingDB Entry DOI: 10.7270/Q2GT5MRF |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50479082

(CHEMBL497501)Show SMILES OC(=O)C(\O)=C\C(=O)c1ccc2n(Cc3ccc(F)cc3)cc(C(=O)\C=C(/O)C(O)=O)c(=O)c2c1 Show InChI InChI=1S/C24H16FNO9/c25-14-4-1-12(2-5-14)10-26-11-16(19(28)9-21(30)24(34)35)22(31)15-7-13(3-6-17(15)26)18(27)8-20(29)23(32)33/h1-9,11,29-30H,10H2,(H,32,33)(H,34,35)/b20-8-,21-9- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Cenci BolognettiUniversit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase strand transfer activity by gel-based assay in presence of magnesium |
J Med Chem 51: 4744-50 (2008)
Article DOI: 10.1021/jm8001422
BindingDB Entry DOI: 10.7270/Q2GM8B3P |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50479098
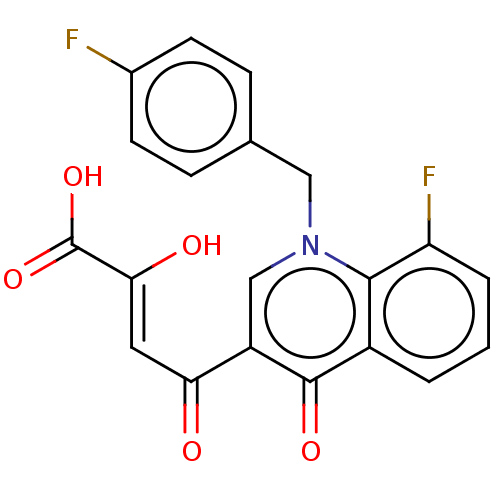
(CHEMBL498698)Show SMILES OC(=O)C(\O)=C\C(=O)c1cn(Cc2ccc(F)cc2)c2c(F)cccc2c1=O Show InChI InChI=1S/C20H13F2NO5/c21-12-6-4-11(5-7-12)9-23-10-14(16(24)8-17(25)20(27)28)19(26)13-2-1-3-15(22)18(13)23/h1-8,10,25H,9H2,(H,27,28)/b17-8- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Cenci BolognettiUniversit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase strand transfer activity by high throughput electrochemiluminescent assay in presence of magnesium |
J Med Chem 51: 4744-50 (2008)
Article DOI: 10.1021/jm8001422
BindingDB Entry DOI: 10.7270/Q2GM8B3P |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50479086
(CHEMBL497688)Show SMILES OC(=O)C(\O)=C\C(=O)c1cn(Cc2ccc(F)cc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C20H13ClFNO5/c21-12-3-6-14-16(7-12)23(9-11-1-4-13(22)5-2-11)10-15(19(14)26)17(24)8-18(25)20(27)28/h1-8,10,25H,9H2,(H,27,28)/b18-8- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Cenci BolognettiUniversit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 integrase strand transfer activity by high throughput electrochemiluminescent assay in presence of magnesium |
J Med Chem 51: 4744-50 (2008)
Article DOI: 10.1021/jm8001422
BindingDB Entry DOI: 10.7270/Q2GM8B3P |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50080949
((S)-2-[4-(3-Phenyl-propoxy)-benzylamino]-propionam...)Show InChI InChI=1S/C19H24N2O2/c1-15(19(20)22)21-14-17-9-11-18(12-10-17)23-13-5-8-16-6-3-2-4-7-16/h2-4,6-7,9-12,15,21H,5,8,13-14H2,1H3,(H2,20,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia& Upjohn
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pentazocine binding to Opioid receptor sigma 1 |
Bioorg Med Chem Lett 9: 2521-4 (1999)
BindingDB Entry DOI: 10.7270/Q29887HT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481188

(CHEMBL571993)Show SMILES CN1CCN(CC1)c1cccc(c1)C(=O)\C=C\c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C23H25N3O3/c1-25-12-14-26(15-13-25)21-7-3-6-20(17-21)22(27)10-8-18-4-2-5-19(16-18)9-11-23(28)24-29/h2-11,16-17,29H,12-15H2,1H3,(H,24,28)/b10-8+,11-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Congenia s.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC extracted from human HeLa cells |
J Med Chem 53: 822-39 (2010)
Article DOI: 10.1021/jm901502p
BindingDB Entry DOI: 10.7270/Q2CR5X5X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data