

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105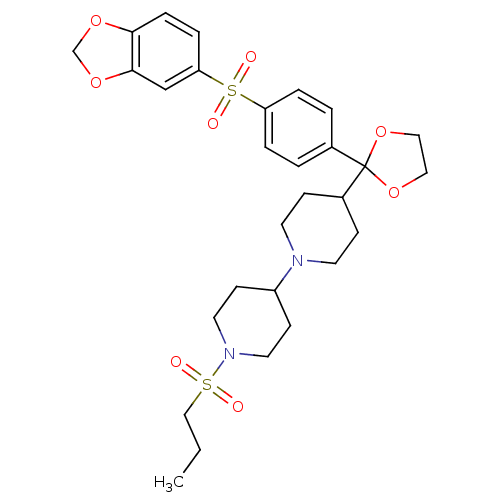 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103771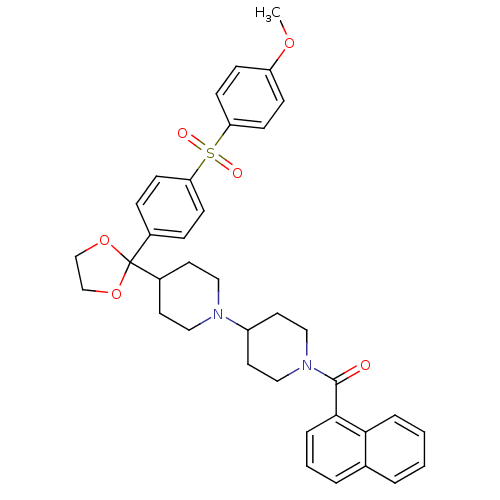 ((4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103774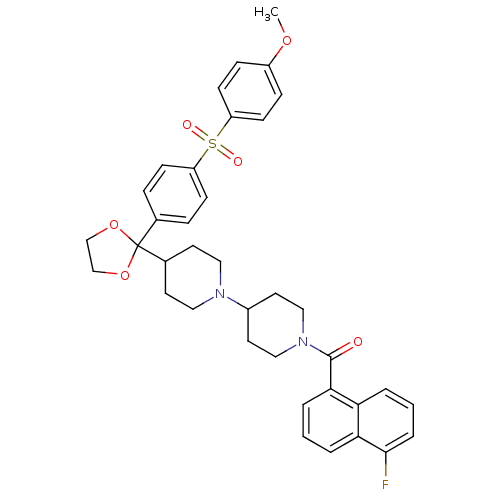 ((5-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103772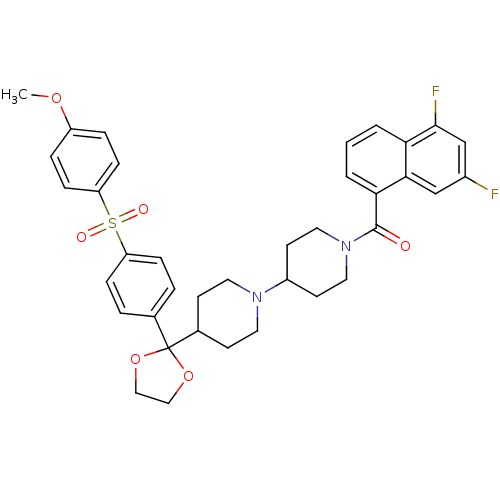 ((5,7-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103767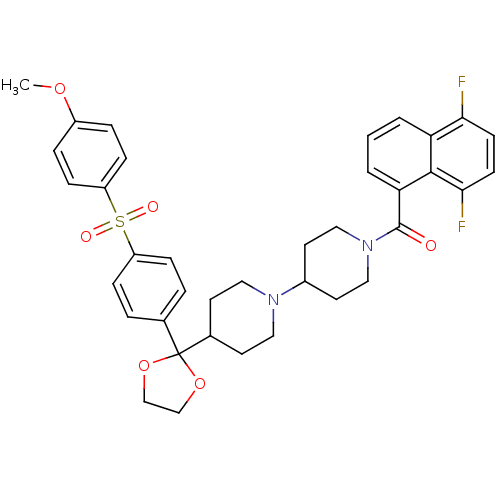 ((5,8-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103773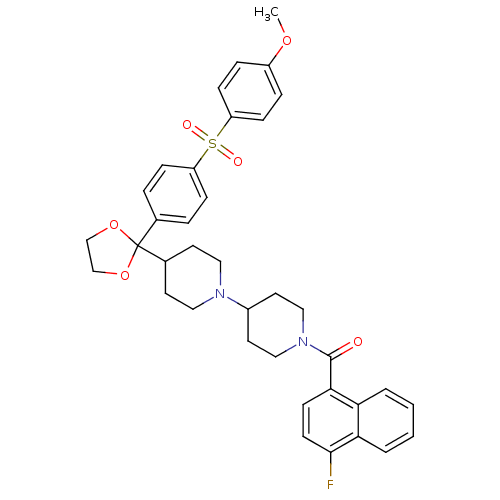 ((4-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103773 ((4-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103768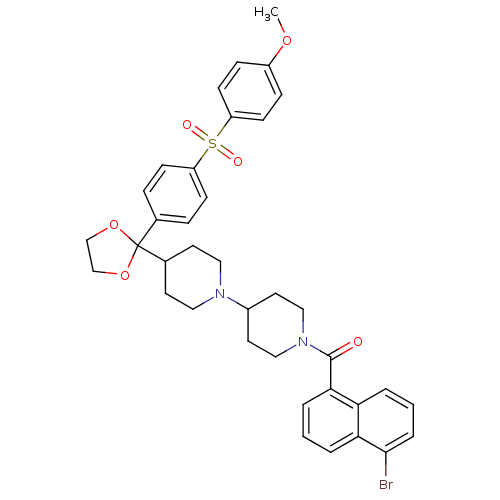 ((5-Bromo-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103775 ((6-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50111346 ((2-Amino-3-methyl-phenyl)-{4-[4-(3-chloro-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50111332 (CHEMBL276239 | [4-(4-Isopropylsulfanyl-benzyl)-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103769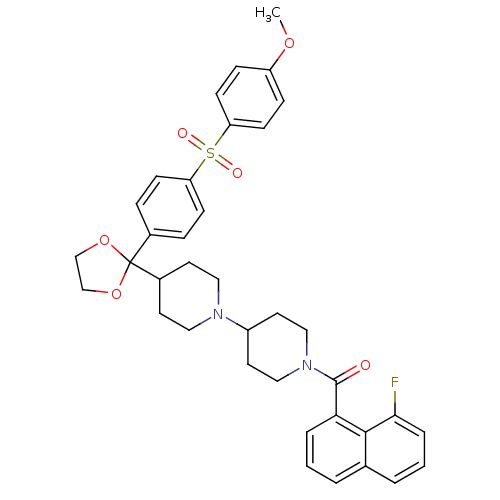 ((8-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103770 ((7-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103776 ((6-Chloro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121488 ((4-Fluoro-naphthalen-1-yl)-(4-{1-[4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121488 ((4-Fluoro-naphthalen-1-yl)-(4-{1-[4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121488 ((4-Fluoro-naphthalen-1-yl)-(4-{1-[4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM35938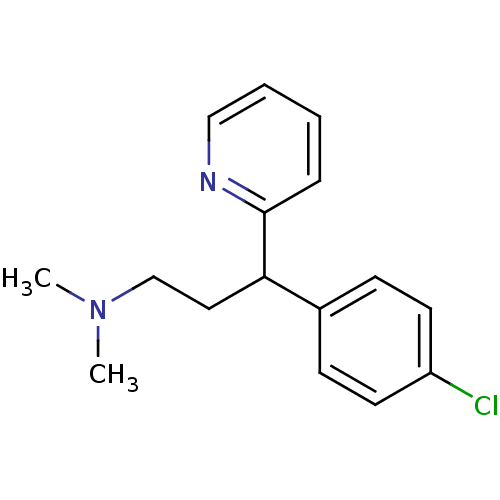 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50063449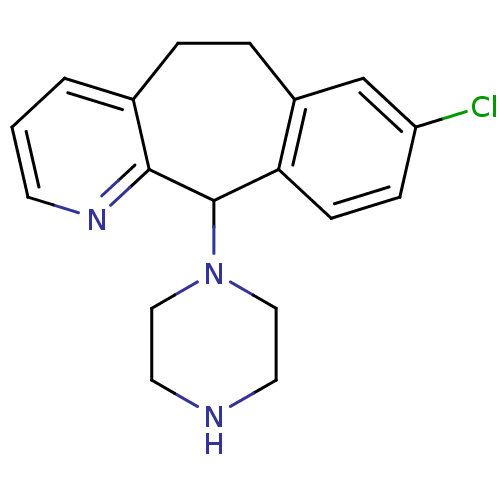 (8-Chloro-11-piperazin-1-yl-6,11-dihydro-5H-benzo[5...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073184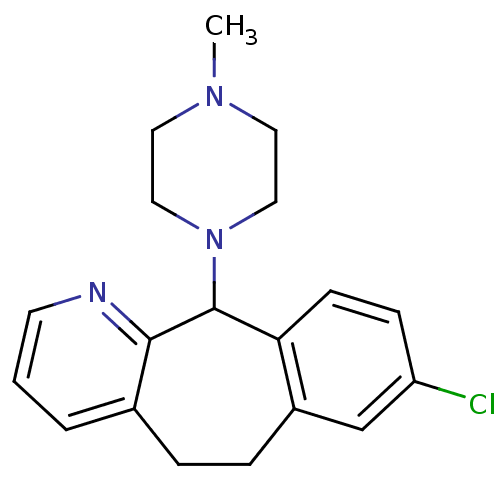 (8-Chloro-11-(4-methyl-piperazin-1-yl)-6,11-dihydro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121489 ((2-Amino-3-methyl-phenyl)-(4-{1-[4-(propane-2-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121492 ((4-Fluoro-naphthalen-1-yl)-(4-{1-[4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121489 ((2-Amino-3-methyl-phenyl)-(4-{1-[4-(propane-2-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121489 ((2-Amino-3-methyl-phenyl)-(4-{1-[4-(propane-2-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121491 ((2-Amino-3-methyl-phenyl)-(4-{1-[4-(propane-2-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103777 (4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073186 (4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50111358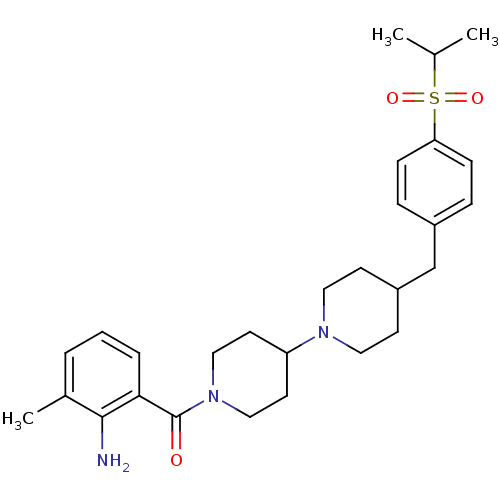 ((2-Amino-3-methyl-phenyl)-{4-[4-(propane-2-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073172 (4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50121490 ((4-Fluoro-naphthalen-1-yl)-{4-[4-(propane-2-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity for human Muscarinic acetylcholine receptor M2 | J Med Chem 45: 5415-8 (2002) BindingDB Entry DOI: 10.7270/Q2ST7P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073180 (4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50007473 (1-[4-(8-Chloro-5,6-dihydro-benzo[5,6]cyclohepta[1,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073176 (1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073182 (1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50403681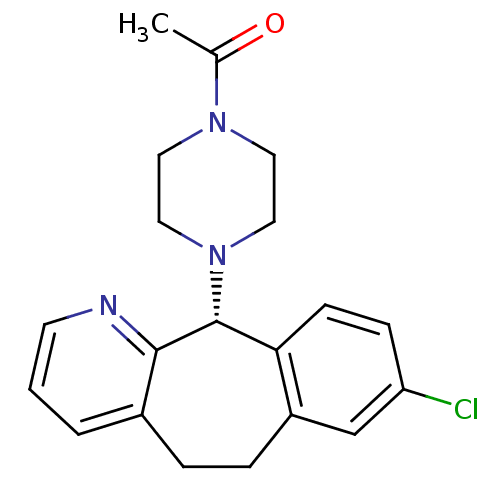 (CHEMBL2111767) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073185 (2-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073175 (1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073181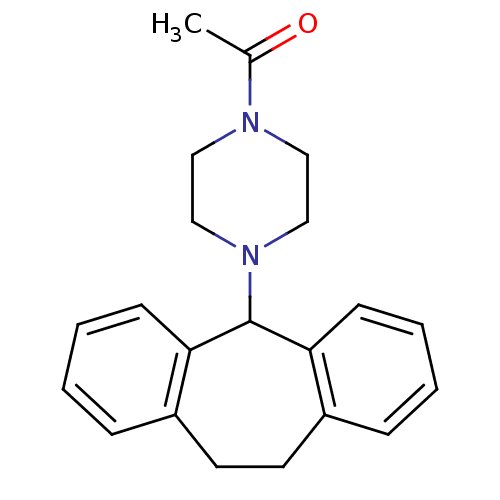 (1-[4-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50073173 (1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50450777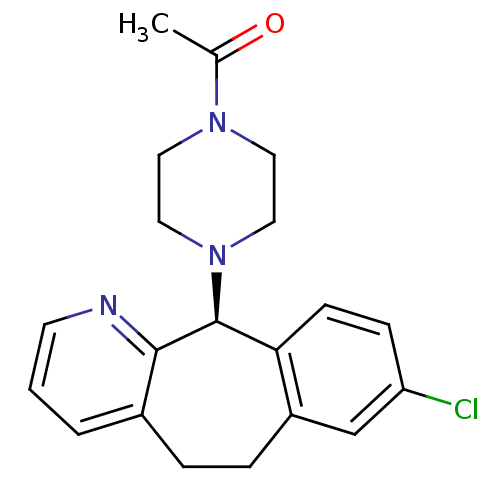 (CHEMBL2448125) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Histamine H1 receptor using receptor binding assay in rat brain membranes | Bioorg Med Chem Lett 8: 3469-74 (1999) BindingDB Entry DOI: 10.7270/Q21J98X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50292221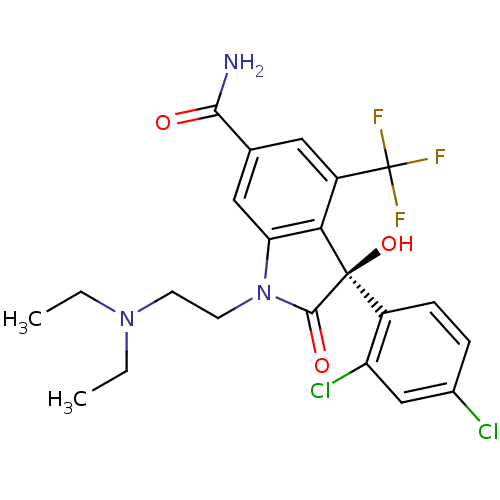 ((S)-3-(2,4-Dichloro-phenyl)-1-(2-diethylamino-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50366689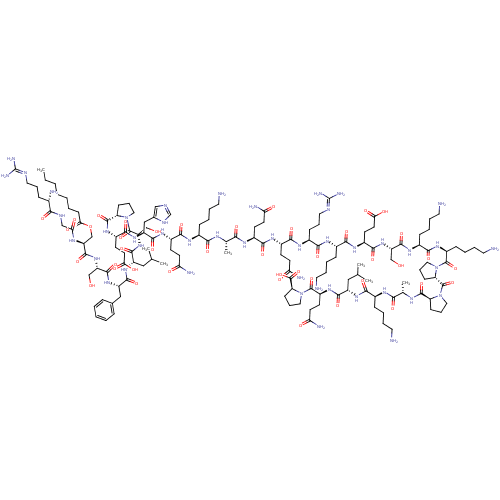 (GHRELIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50292225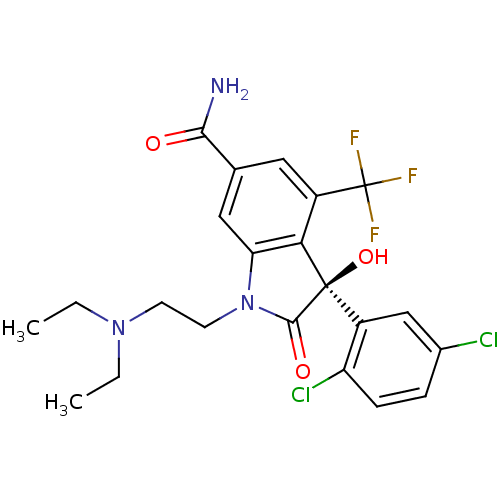 ((S)-3-(2,5-Dichloro-phenyl)-1-(2-diethylamino-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.721 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50292228 ((S)-3-(2,3-Dichloro-phenyl)-1-(2-diethylamino-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151162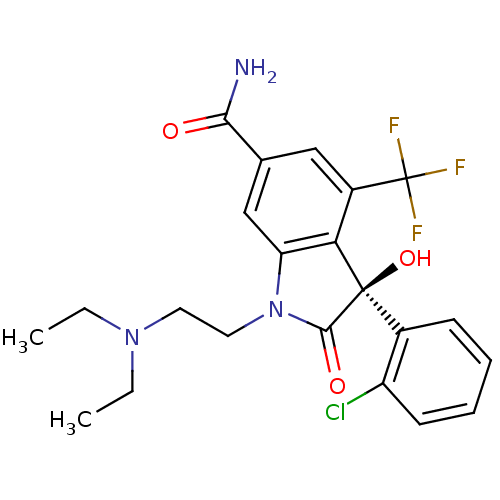 ((S)-3-(2-Chloro-phenyl)-1-(2-diethylamino-ethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50492297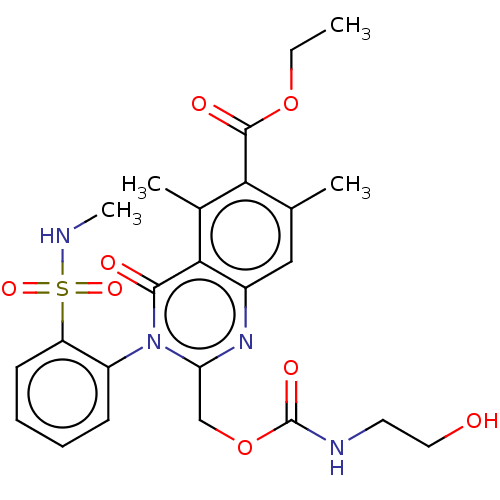 (CHEMBL2397751) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at CB2 receptor (unknown origin) | J Med Chem 56: 5464-72 (2013) Article DOI: 10.1021/jm4004939 BindingDB Entry DOI: 10.7270/Q2833VZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50151162 ((S)-3-(2-Chloro-phenyl)-1-(2-diethylamino-ethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50292230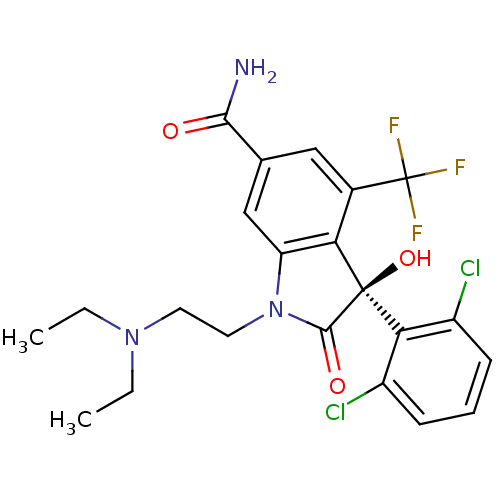 ((S)-3-(2,6-Dichloro-phenyl)-1-(2-diethylamino-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation | Bioorg Med Chem Lett 15: 1789-92 (2005) Article DOI: 10.1016/j.bmcl.2005.02.042 BindingDB Entry DOI: 10.7270/Q24J0FWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 269 total ) | Next | Last >> |