

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076548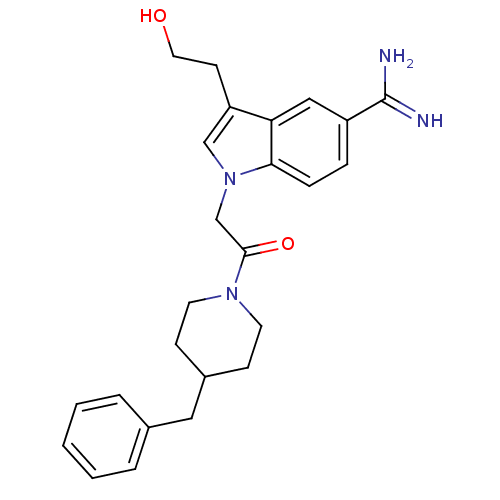 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-3-(2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076545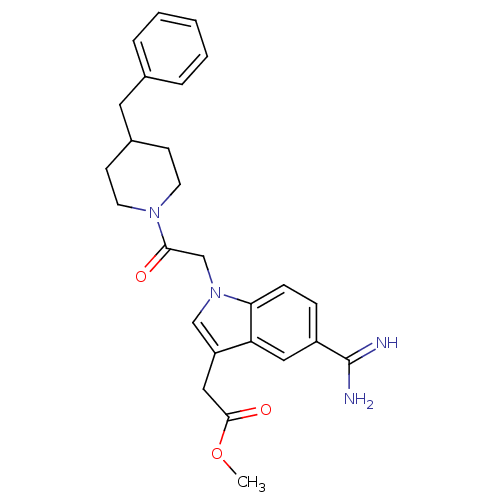 (CHEMBL422722 | {1-[2-(4-Benzyl-piperidin-1-yl)-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076540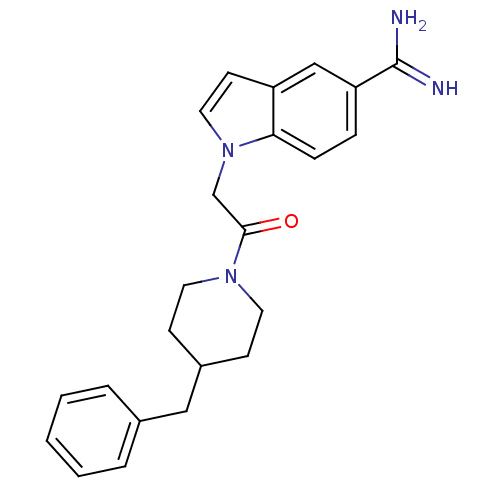 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-1H-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076549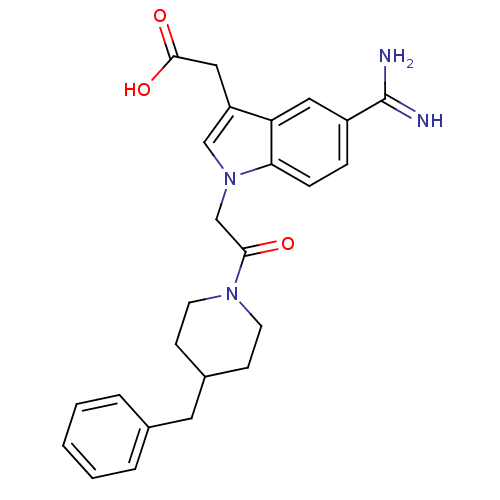 (CHEMBL366643 | {1-[2-(4-Benzyl-piperidin-1-yl)-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076547 (1-{2-[4-(2-Fluoro-benzyl)-piperidin-1-yl]-2-oxo-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076538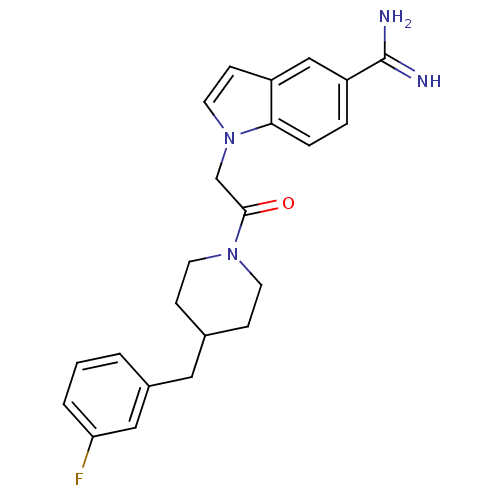 (1-{2-[4-(3-Fluoro-benzyl)-piperidin-1-yl]-2-oxo-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076537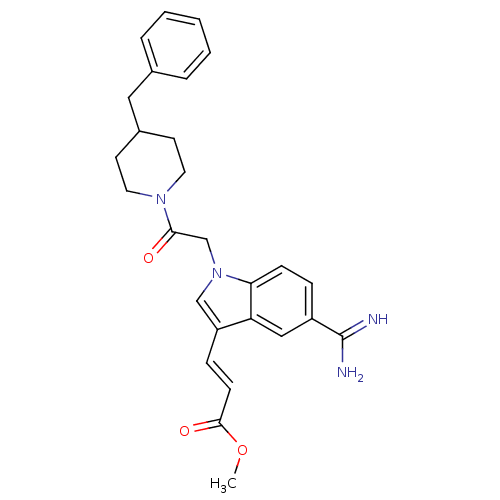 (3-{1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076545 (CHEMBL422722 | {1-[2-(4-Benzyl-piperidin-1-yl)-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076546 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-1H-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076542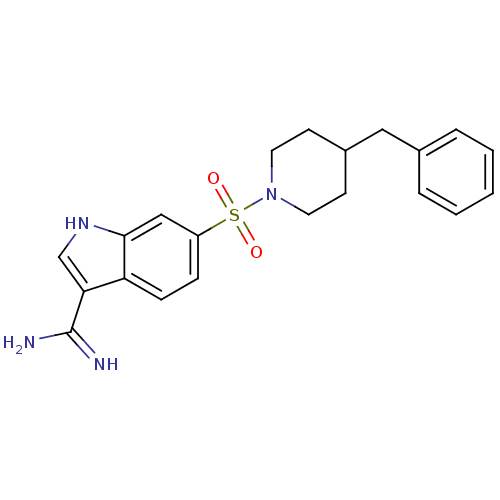 (6-(4-Benzyl-piperidine-1-sulfonyl)-1H-indole-3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076537 (3-{1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076539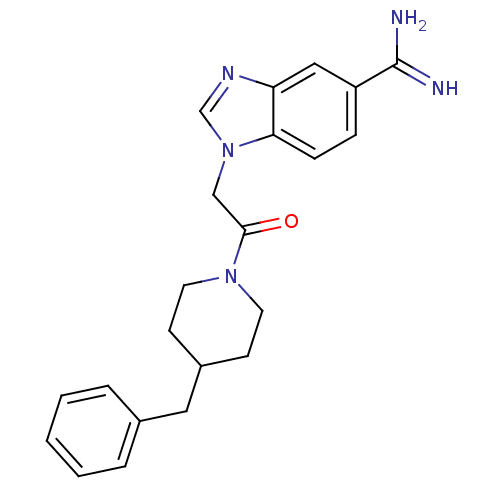 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-1H-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076548 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-3-(2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076542 (6-(4-Benzyl-piperidine-1-sulfonyl)-1H-indole-3-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076546 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076543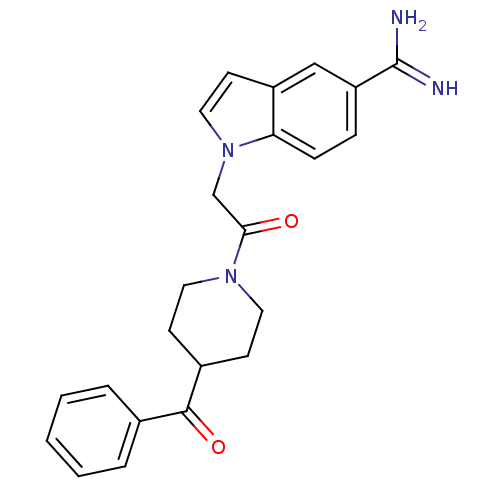 (1-[2-(4-Benzoyl-piperidin-1-yl)-2-oxo-ethyl]-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076540 (1-[2-(4-Benzyl-piperidin-1-yl)-2-oxo-ethyl]-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50076541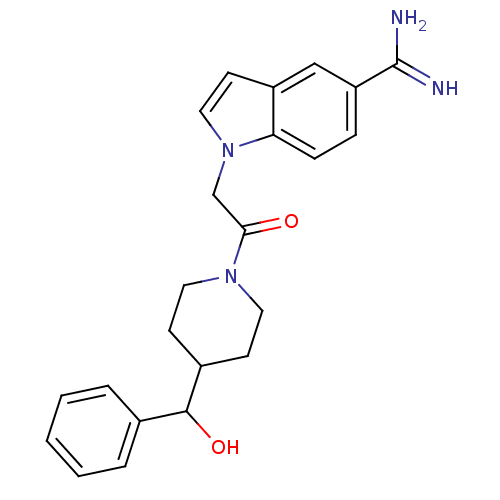 (1-{2-[4-(Hydroxy-phenyl-methyl)-piperidin-1-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076541 (1-{2-[4-(Hydroxy-phenyl-methyl)-piperidin-1-yl]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076543 (1-[2-(4-Benzoyl-piperidin-1-yl)-2-oxo-ethyl]-1H-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||