 Found 678 hits with Last Name = 'an' and Initial = 'sq'
Found 678 hits with Last Name = 'an' and Initial = 'sq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50457437
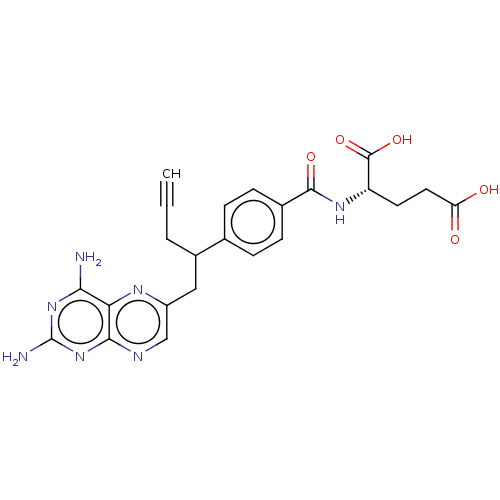
(CHEBI:71223 | Folotyn | PDX | Pralatrexate)Show SMILES [H][C@@](CCC(O)=O)(NC(=O)c1ccc(cc1)C(CC#C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O |r| Show InChI InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50027656
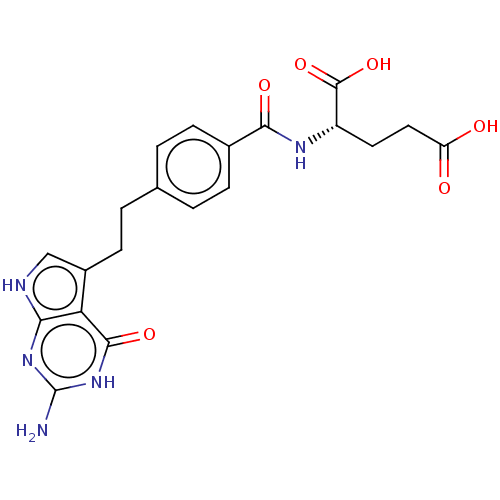
(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50027656

(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... |
Eur J Med Chem 157: 161-176 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.005
BindingDB Entry DOI: 10.7270/Q29P34C1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50027656

(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50469009
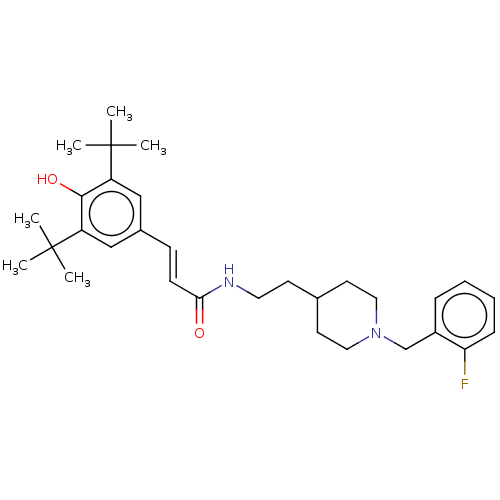
(CHEMBL4292766)Show SMILES CC(C)(C)c1cc(\C=C\C(=O)NCCC2CCN(Cc3ccccc3F)CC2)cc(c1O)C(C)(C)C Show InChI InChI=1S/C31H43FN2O2/c1-30(2,3)25-19-23(20-26(29(25)36)31(4,5)6)11-12-28(35)33-16-13-22-14-17-34(18-15-22)21-24-9-7-8-10-27(24)32/h7-12,19-20,22,36H,13-18,21H2,1-6H3,(H,33,35)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... |
Eur J Med Chem 157: 161-176 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.005
BindingDB Entry DOI: 10.7270/Q29P34C1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50469008
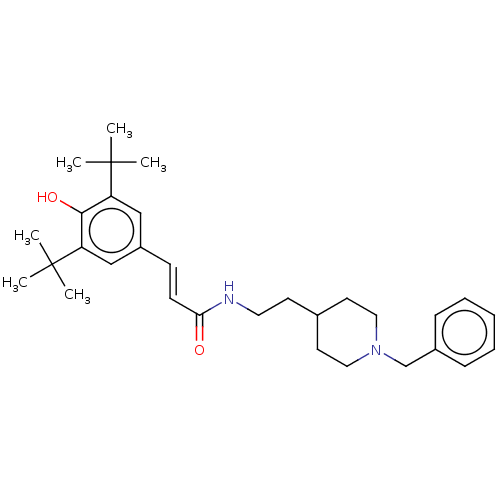
(CHEMBL4283390)Show SMILES CC(C)(C)c1cc(\C=C\C(=O)NCCC2CCN(Cc3ccccc3)CC2)cc(c1O)C(C)(C)C Show InChI InChI=1S/C31H44N2O2/c1-30(2,3)26-20-25(21-27(29(26)35)31(4,5)6)12-13-28(34)32-17-14-23-15-18-33(19-16-23)22-24-10-8-7-9-11-24/h7-13,20-21,23,35H,14-19,22H2,1-6H3,(H,32,34)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... |
Eur J Med Chem 157: 161-176 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.005
BindingDB Entry DOI: 10.7270/Q29P34C1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502319
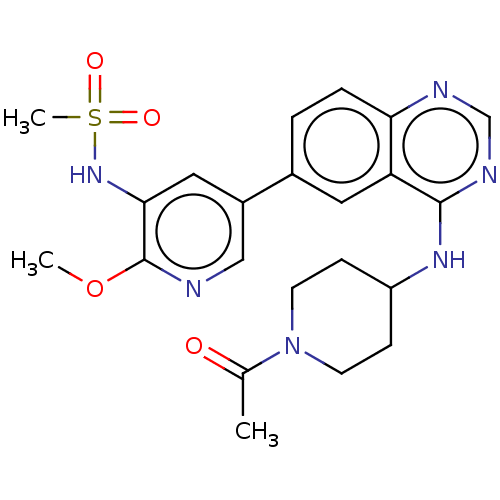
(CHEMBL4515169)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C22H26N6O4S/c1-14(29)28-8-6-17(7-9-28)26-21-18-10-15(4-5-19(18)24-13-25-21)16-11-20(27-33(3,30)31)22(32-2)23-12-16/h4-5,10-13,17,27H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502315
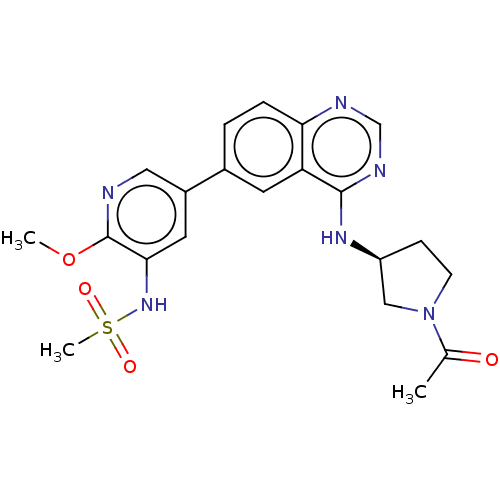
(CHEMBL4449091)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(C)=O)c2c1 |r| Show InChI InChI=1S/C21H24N6O4S/c1-13(28)27-7-6-16(11-27)25-20-17-8-14(4-5-18(17)23-12-24-20)15-9-19(26-32(3,29)30)21(31-2)22-10-15/h4-5,8-10,12,16,26H,6-7,11H2,1-3H3,(H,23,24,25)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502318
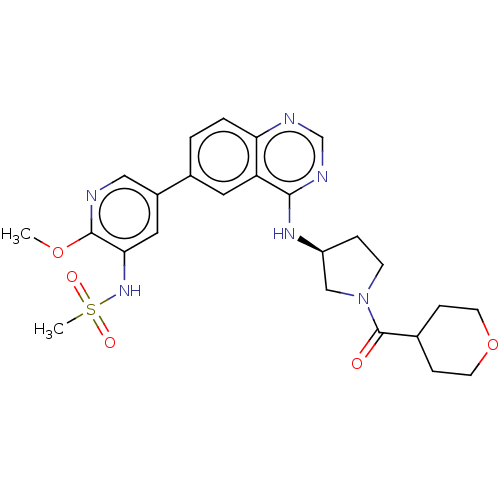
(CHEMBL4542688)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2c1 |r| Show InChI InChI=1S/C25H30N6O5S/c1-35-24-22(30-37(2,33)34)12-18(13-26-24)17-3-4-21-20(11-17)23(28-15-27-21)29-19-5-8-31(14-19)25(32)16-6-9-36-10-7-16/h3-4,11-13,15-16,19,30H,5-10,14H2,1-2H3,(H,27,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50525693
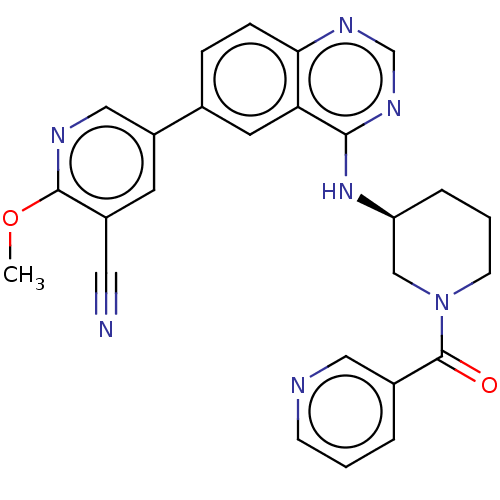
(CHEMBL4468088)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(N[C@H]3CCCN(C3)C(=O)c3cccnc3)c2c1 |r| Show InChI InChI=1S/C26H23N7O2/c1-35-25-19(12-27)10-20(14-29-25)17-6-7-23-22(11-17)24(31-16-30-23)32-21-5-3-9-33(15-21)26(34)18-4-2-8-28-13-18/h2,4,6-8,10-11,13-14,16,21H,3,5,9,15H2,1H3,(H,30,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K delta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458067
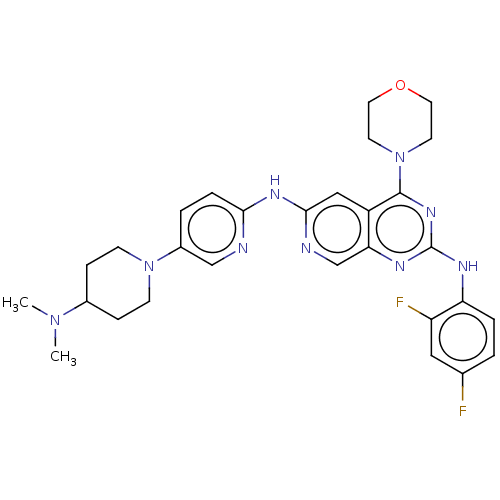
(CHEMBL4215080)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4F)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H33F2N9O/c1-38(2)20-7-9-39(10-8-20)21-4-6-26(32-17-21)36-27-16-22-25(18-33-27)35-29(34-24-5-3-19(30)15-23(24)31)37-28(22)40-11-13-41-14-12-40/h3-6,15-18,20H,7-14H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458066
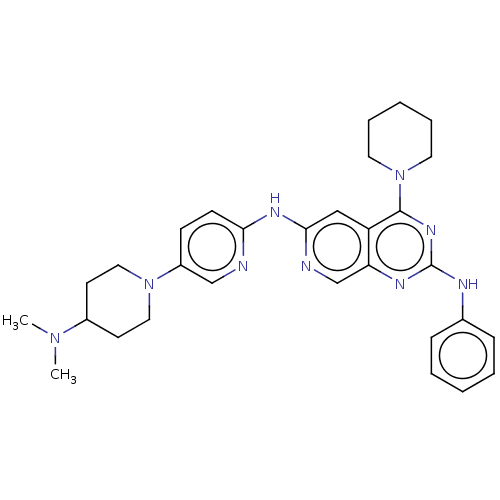
(CHEMBL4203016)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C30H37N9/c1-37(2)23-13-17-38(18-14-23)24-11-12-27(31-20-24)35-28-19-25-26(21-32-28)34-30(33-22-9-5-3-6-10-22)36-29(25)39-15-7-4-8-16-39/h3,5-6,9-12,19-21,23H,4,7-8,13-18H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502321
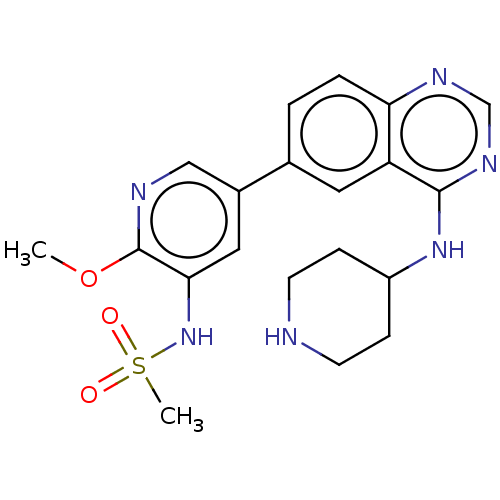
(CHEMBL4461362)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCNCC3)c2c1 Show InChI InChI=1S/C20H24N6O3S/c1-29-20-18(26-30(2,27)28)10-14(11-22-20)13-3-4-17-16(9-13)19(24-12-23-17)25-15-5-7-21-8-6-15/h3-4,9-12,15,21,26H,5-8H2,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502317
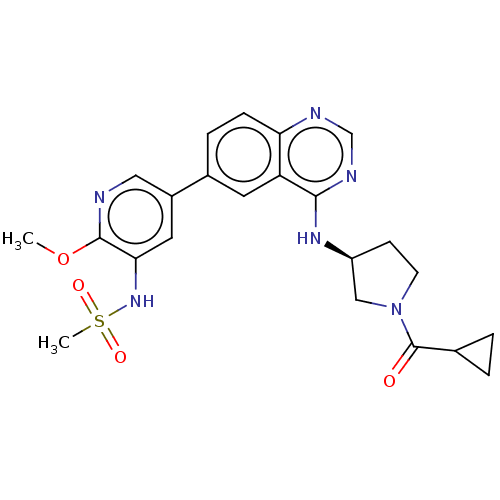
(CHEMBL4471468)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(N[C@H]3CCN(C3)C(=O)C3CC3)c2c1 |r| Show InChI InChI=1S/C23H26N6O4S/c1-33-22-20(28-34(2,31)32)10-16(11-24-22)15-5-6-19-18(9-15)21(26-13-25-19)27-17-7-8-29(12-17)23(30)14-3-4-14/h5-6,9-11,13-14,17,28H,3-4,7-8,12H2,1-2H3,(H,25,26,27)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T790M double mutant (unknown origin) |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458062
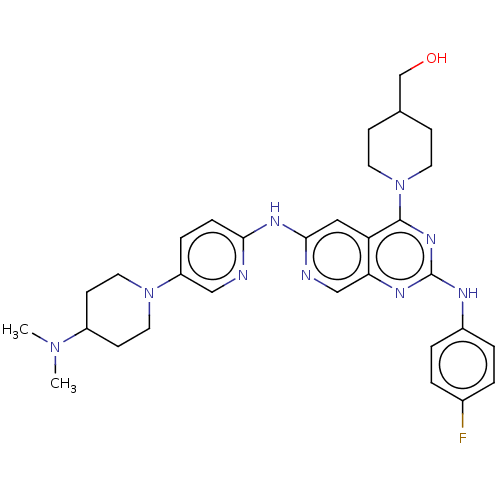
(CHEMBL4209019)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCC(CO)CC2)nc1 Show InChI InChI=1S/C31H38FN9O/c1-39(2)24-11-15-40(16-12-24)25-7-8-28(33-18-25)37-29-17-26-27(19-34-29)36-31(35-23-5-3-22(32)4-6-23)38-30(26)41-13-9-21(20-42)10-14-41/h3-8,17-19,21,24,42H,9-16,20H2,1-2H3,(H,33,34,37)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068
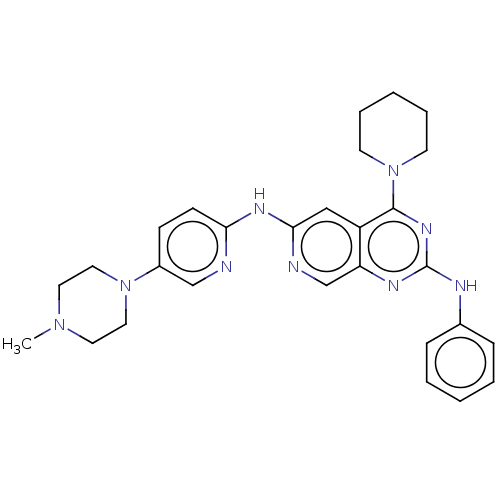
(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50485688
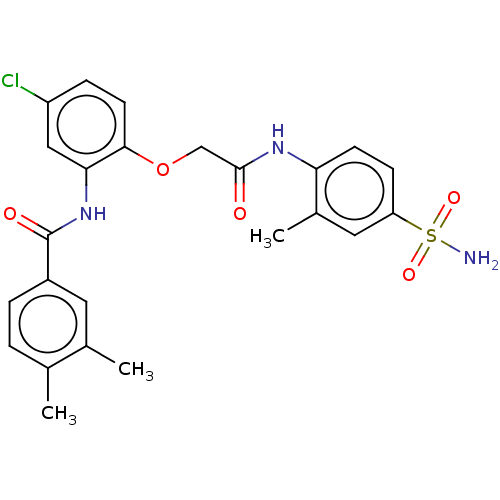
(CHEMBL2151836)Show SMILES Cc1ccc(cc1C)C(=O)Nc1cc(Cl)ccc1OCC(=O)Nc1ccc(cc1C)S(N)(=O)=O Show InChI InChI=1S/C24H24ClN3O5S/c1-14-4-5-17(10-15(14)2)24(30)28-21-12-18(25)6-9-22(21)33-13-23(29)27-20-8-7-19(11-16(20)3)34(26,31)32/h4-12H,13H2,1-3H3,(H,27,29)(H,28,30)(H2,26,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA |
Eur J Med Chem 58: 504-12 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.032
BindingDB Entry DOI: 10.7270/Q2K07747 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068

(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50461172
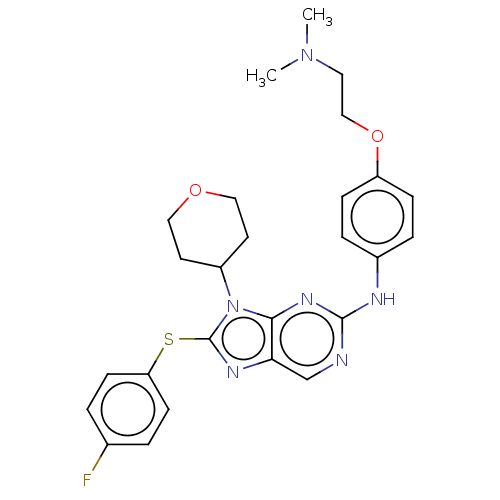
(CHEMBL4228518)Show SMILES CN(C)CCOc1ccc(Nc2ncc3nc(Sc4ccc(F)cc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C26H29FN6O2S/c1-32(2)13-16-35-21-7-5-19(6-8-21)29-25-28-17-23-24(31-25)33(20-11-14-34-15-12-20)26(30-23)36-22-9-3-18(27)4-10-22/h3-10,17,20H,11-16H2,1-2H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
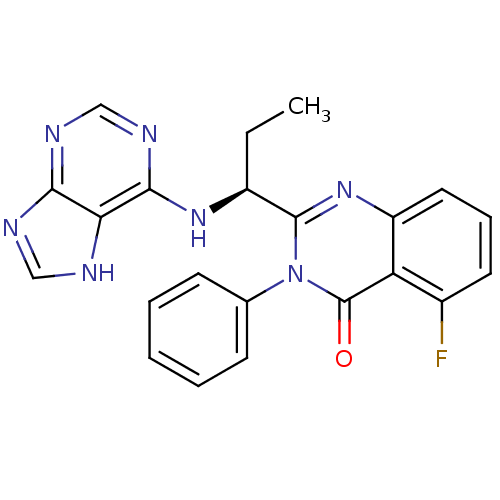
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K delta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR-2 (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50525687
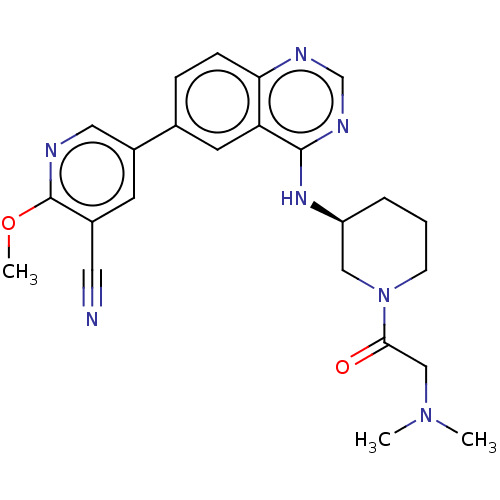
(CHEMBL4476303)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(N[C@H]3CCCN(C3)C(=O)CN(C)C)c2c1 |r| Show InChI InChI=1S/C24H27N7O2/c1-30(2)14-22(32)31-8-4-5-19(13-31)29-23-20-10-16(6-7-21(20)27-15-28-23)18-9-17(11-25)24(33-3)26-12-18/h6-7,9-10,12,15,19H,4-5,8,13-14H2,1-3H3,(H,27,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K delta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR tyrosine kinase (unknown origin) assessed as ATP level by luminescence analysis |
Bioorg Med Chem 24: 179-90 (2016)
Article DOI: 10.1016/j.bmc.2015.12.001
BindingDB Entry DOI: 10.7270/Q2SJ1PM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458060
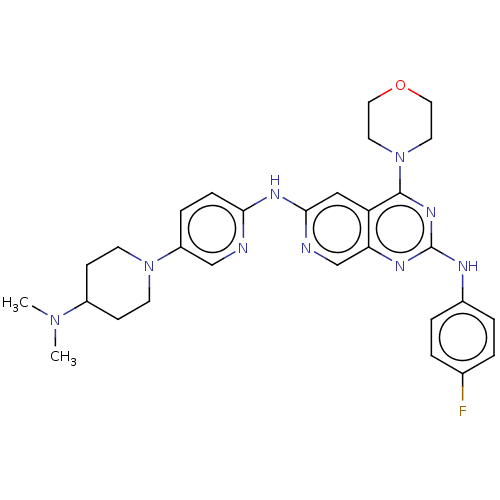
(CHEMBL4215076)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H34FN9O/c1-37(2)22-9-11-38(12-10-22)23-7-8-26(31-18-23)35-27-17-24-25(19-32-27)34-29(33-21-5-3-20(30)4-6-21)36-28(24)39-13-15-40-16-14-39/h3-8,17-19,22H,9-16H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50274957
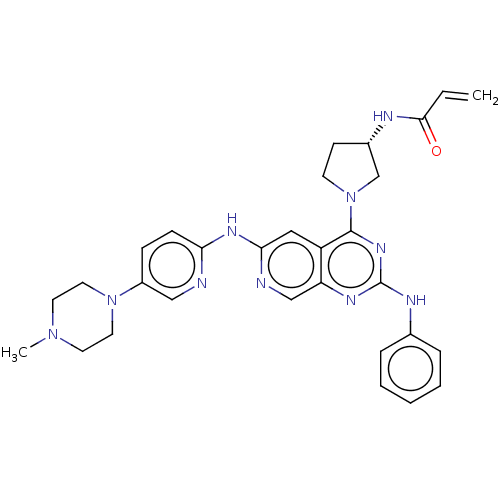
(CHEMBL4127809)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CC[C@@H](C2)NC(=O)C=C)nc1 |r| Show InChI InChI=1S/C30H34N10O/c1-3-28(41)33-22-11-12-40(20-22)29-24-17-27(32-19-25(24)35-30(37-29)34-21-7-5-4-6-8-21)36-26-10-9-23(18-31-26)39-15-13-38(2)14-16-39/h3-10,17-19,22H,1,11-16,20H2,2H3,(H,33,41)(H,31,32,36)(H,34,35,37)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50461174
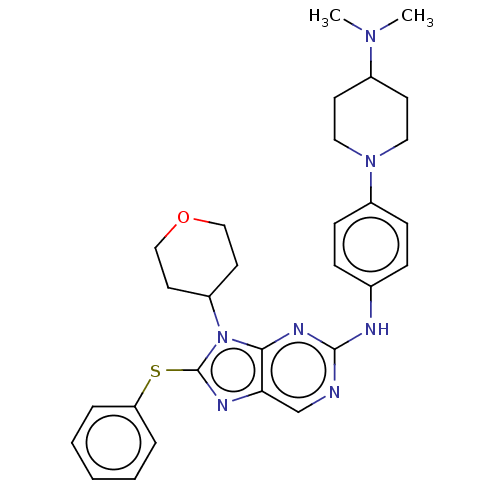
(CHEMBL4226676)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2ncc3nc(Sc4ccccc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C29H35N7OS/c1-34(2)22-12-16-35(17-13-22)23-10-8-21(9-11-23)31-28-30-20-26-27(33-28)36(24-14-18-37-19-15-24)29(32-26)38-25-6-4-3-5-7-25/h3-11,20,22,24H,12-19H2,1-2H3,(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458065
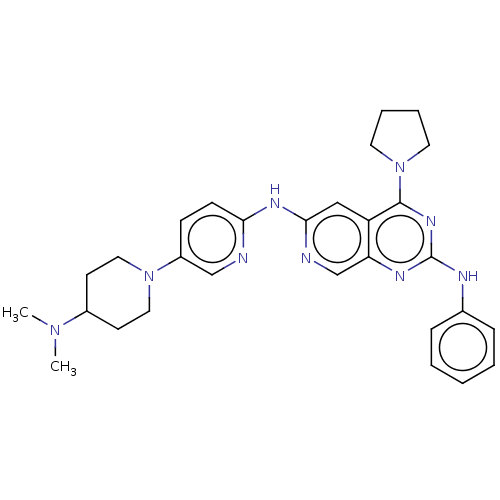
(CHEMBL4212884)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCC2)nc1 Show InChI InChI=1S/C29H35N9/c1-36(2)22-12-16-37(17-13-22)23-10-11-26(30-19-23)34-27-18-24-25(20-31-27)33-29(32-21-8-4-3-5-9-21)35-28(24)38-14-6-7-15-38/h3-5,8-11,18-20,22H,6-7,12-17H2,1-2H3,(H,30,31,34)(H,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50274925
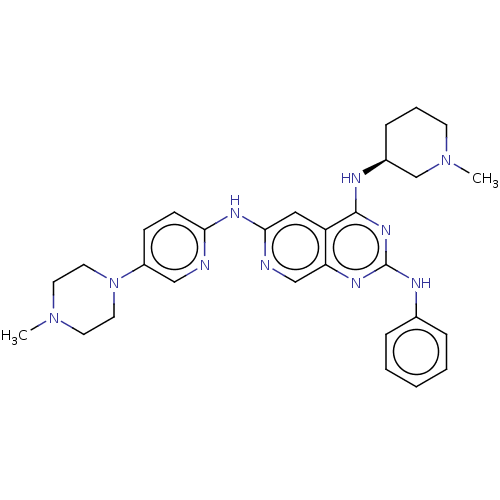
(CHEMBL4126810)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(N[C@H]4CCCN(C)C4)nc(Nc4ccccc4)nc3cn2)nc1 |r| Show InChI InChI=1S/C29H36N10/c1-37-13-15-39(16-14-37)23-10-11-26(30-18-23)35-27-17-24-25(19-31-27)34-29(33-21-7-4-3-5-8-21)36-28(24)32-22-9-6-12-38(2)20-22/h3-5,7-8,10-11,17-19,22H,6,9,12-16,20H2,1-2H3,(H,30,31,35)(H2,32,33,34,36)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50461178
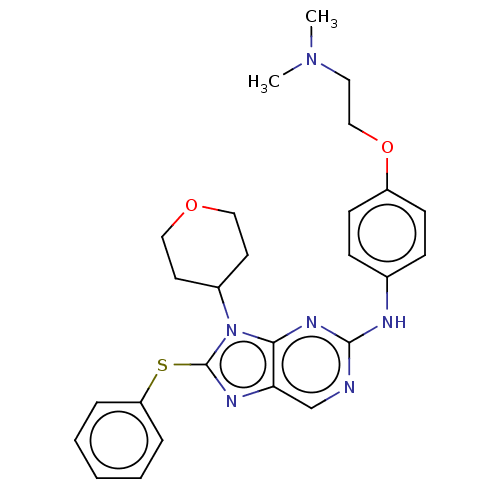
(CHEMBL4225777)Show SMILES CN(C)CCOc1ccc(Nc2ncc3nc(Sc4ccccc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C26H30N6O2S/c1-31(2)14-17-34-21-10-8-19(9-11-21)28-25-27-18-23-24(30-25)32(20-12-15-33-16-13-20)26(29-23)35-22-6-4-3-5-7-22/h3-11,18,20H,12-17H2,1-2H3,(H,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T790M double mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50525697
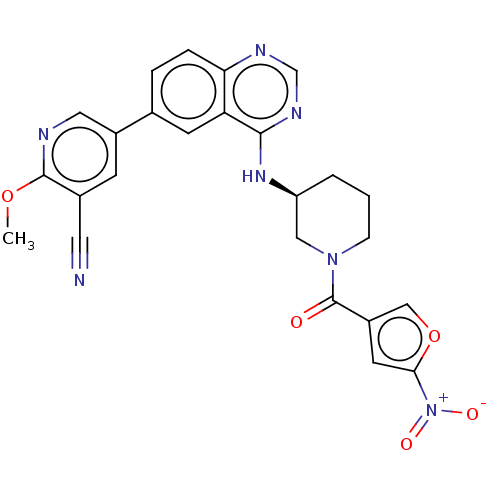
(CHEMBL4457318)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(N[C@H]3CCCN(C3)C(=O)c3coc(c3)[N+]([O-])=O)c2c1 |r| Show InChI InChI=1S/C25H21N7O5/c1-36-24-16(10-26)7-17(11-27-24)15-4-5-21-20(8-15)23(29-14-28-21)30-19-3-2-6-31(12-19)25(33)18-9-22(32(34)35)37-13-18/h4-5,7-9,11,13-14,19H,2-3,6,12H2,1H3,(H,28,29,30)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50274925

(CHEMBL4126810)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(N[C@H]4CCCN(C)C4)nc(Nc4ccccc4)nc3cn2)nc1 |r| Show InChI InChI=1S/C29H36N10/c1-37-13-15-39(16-14-37)23-10-11-26(30-18-23)35-27-17-24-25(19-31-27)34-29(33-21-7-4-3-5-8-21)36-28(24)32-22-9-6-12-38(2)20-22/h3-5,7-8,10-11,17-19,22H,6,9,12-16,20H2,1-2H3,(H,30,31,35)(H2,32,33,34,36)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly(Glu,Tyr) as substrate after 40 mins by Kinase-Glo luminescence assay |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50461174

(CHEMBL4226676)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2ncc3nc(Sc4ccccc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C29H35N7OS/c1-34(2)22-12-16-35(17-13-22)23-10-8-21(9-11-23)31-28-30-20-26-27(33-28)36(24-14-18-37-19-15-24)29(32-26)38-25-6-4-3-5-7-25/h3-11,20,22,24H,12-19H2,1-2H3,(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem 26: 3619-3633 (2018)
Article DOI: 10.1016/j.bmc.2018.05.039
BindingDB Entry DOI: 10.7270/Q2Z3224Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50525693

(CHEMBL4468088)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(N[C@H]3CCCN(C3)C(=O)c3cccnc3)c2c1 |r| Show InChI InChI=1S/C26H23N7O2/c1-35-25-19(12-27)10-20(14-29-25)17-6-7-23-22(11-17)24(31-16-30-23)32-21-5-3-9-33(15-21)26(34)18-4-2-8-28-13-18/h2,4,6-8,10-11,13-14,16,21H,3,5,9,15H2,1H3,(H,30,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K gamma (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins in presence of ATP by Kinase-Glo Plus reage... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.051
BindingDB Entry DOI: 10.7270/Q2MC93GX |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-3
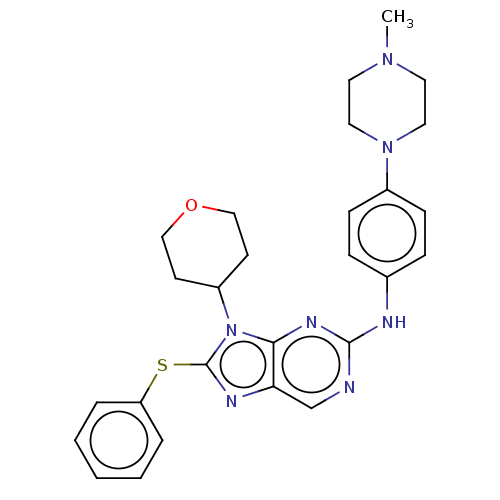
(Homo sapiens (Human)) | BDBM50461179
(CHEMBL4226151)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3nc(Sc4ccccc4)n(C4CCOCC4)c3n2)cc1 Show InChI InChI=1S/C27H31N7OS/c1-32-13-15-33(16-14-32)21-9-7-20(8-10-21)29-26-28-19-24-25(31-26)34(22-11-17-35-18-12-22)27(30-24)36-23-5-3-2-4-6-23/h2-10,19,22H,11-18H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-3
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) using poly (Glu, Tyr) as substrate after 40 mins by kinase-glo plus luminescence assay |
Bioorg Med Chem 26: 2173-2185 (2018)
Article DOI: 10.1016/j.bmc.2018.03.025
BindingDB Entry DOI: 10.7270/Q2H41V28 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
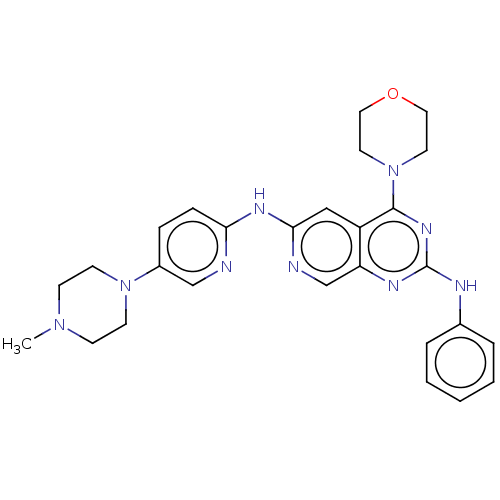
(Homo sapiens (Human)) | BDBM50458063
(CHEMBL4206288)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C27H31N9O/c1-34-9-11-35(12-10-34)21-7-8-24(28-18-21)32-25-17-22-23(19-29-25)31-27(30-20-5-3-2-4-6-20)33-26(22)36-13-15-37-16-14-36/h2-8,17-19H,9-16H2,1H3,(H,28,29,32)(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data