

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236864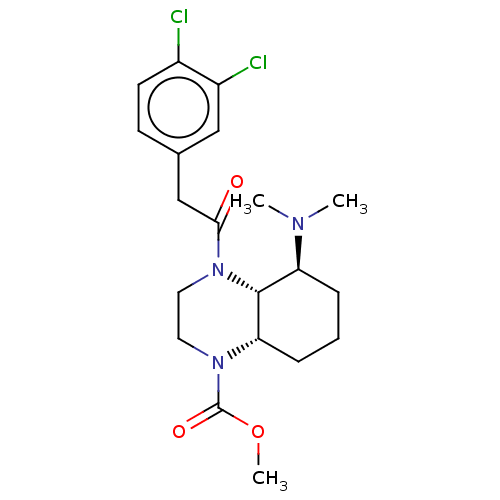 (CHEMBL4096473) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Inhibition of high affinity uptake of [3H]DA using rat nerve endings obtained from brain regions enriched in DAT. | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130757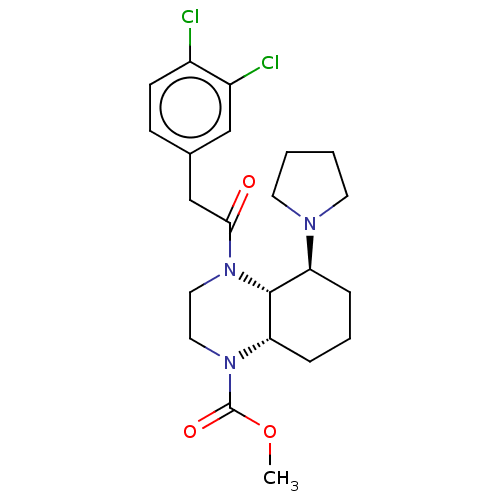 (CHEMBL3634516) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236870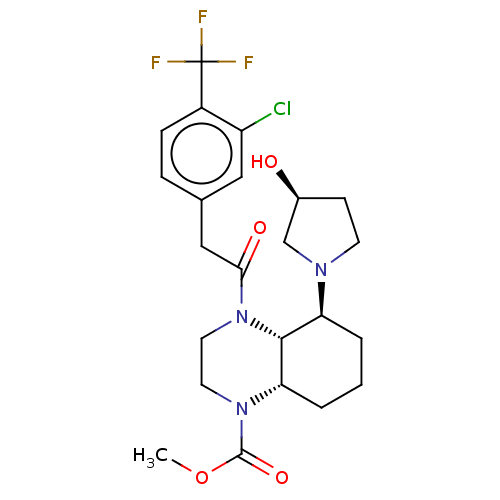 (CHEMBL4064380) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143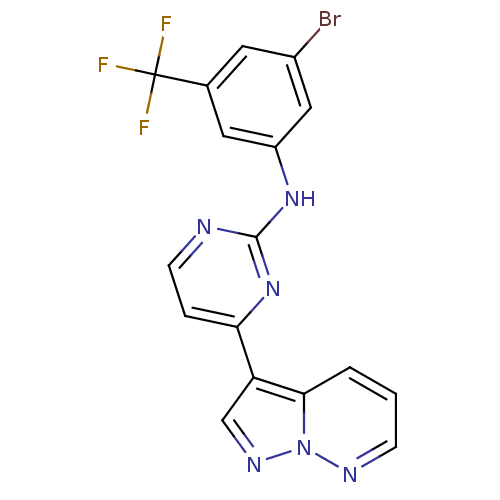 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236878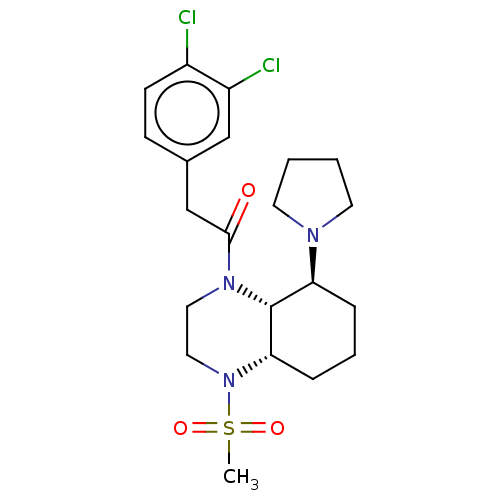 (CHEMBL4084350) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50277608 (CHEMBL4173067) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236871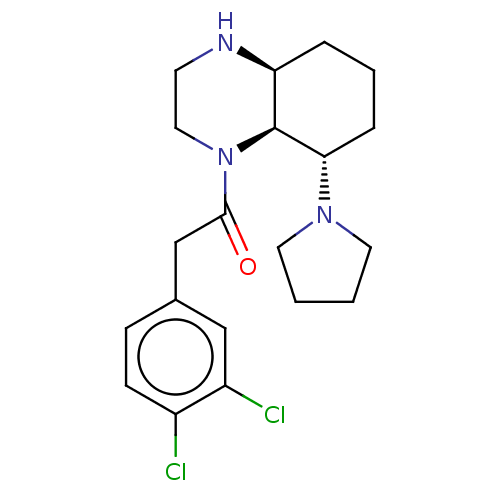 (CHEMBL4069231) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236886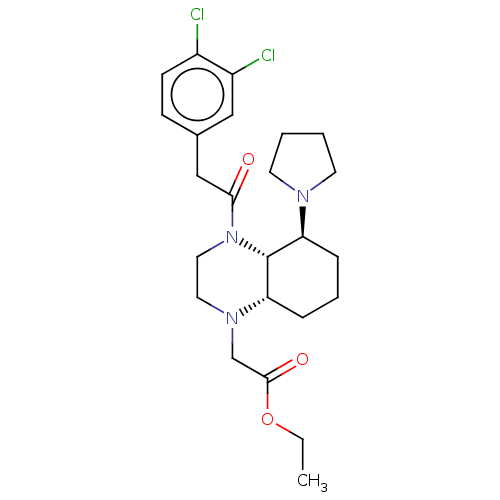 (CHEMBL4099849) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236865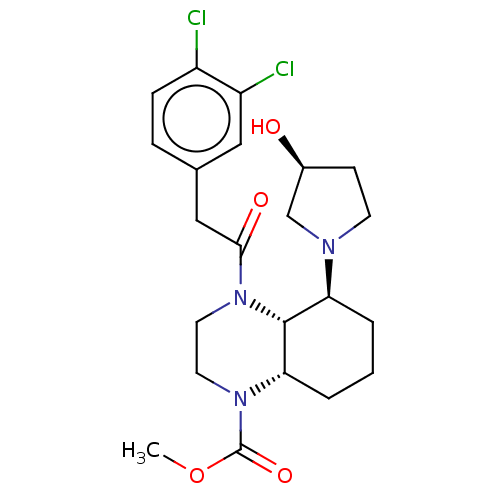 (CHEMBL4095192) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236874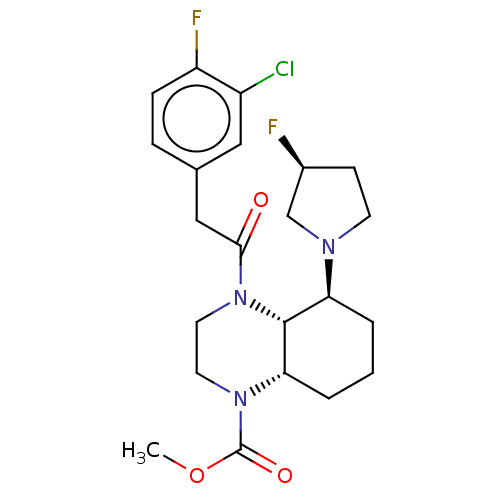 (CHEMBL4072961) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]NE from Norepinephrine transporter of rat brain | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora A kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236875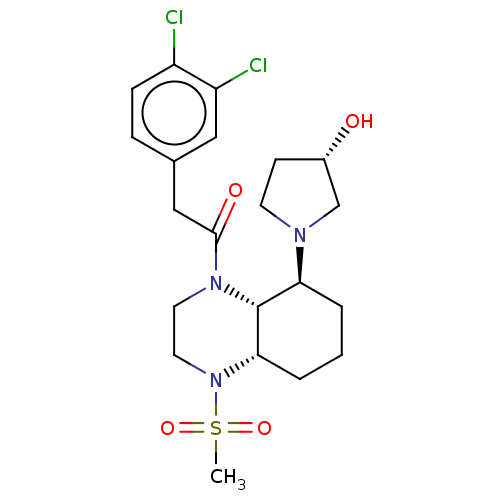 (CHEMBL4074031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring inhibiting the binding of [3H]citalopram to Serotonin transporter in rat brain tissue | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236868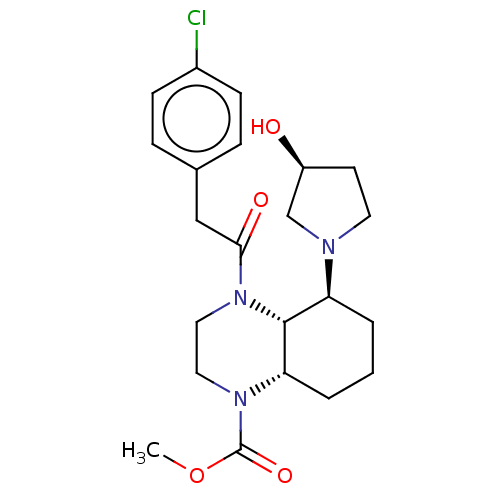 (CHEMBL4091184) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35248 from dopamine transporter (DAT) of rat striatal membrane | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236872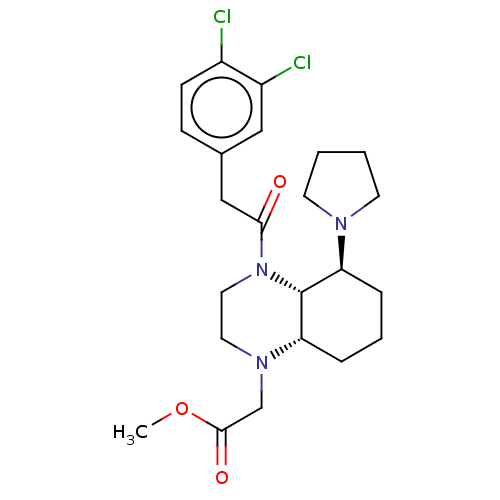 (CHEMBL4072972) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236866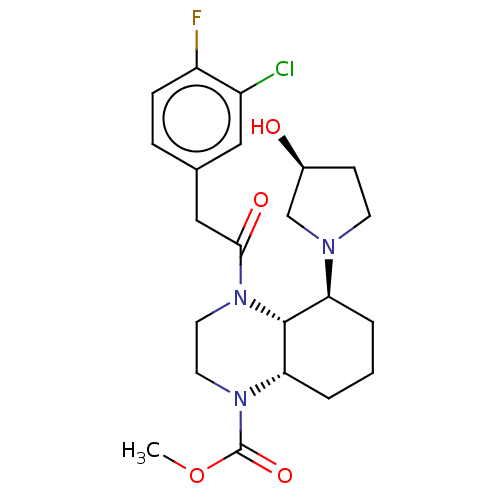 (CHEMBL4061582) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236879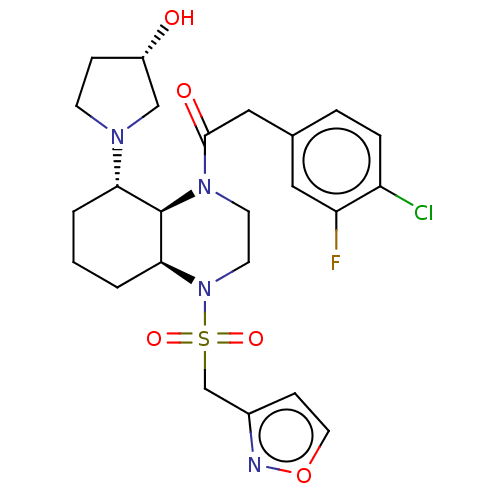 (CHEMBL4069149) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236881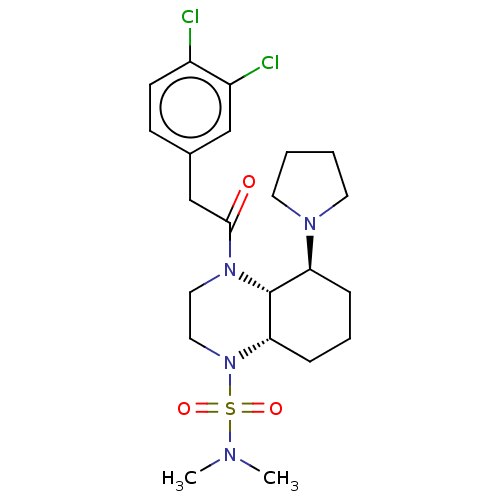 (CHEMBL4094571) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21130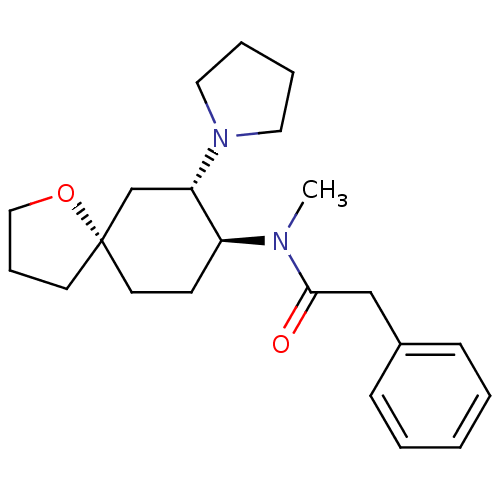 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236882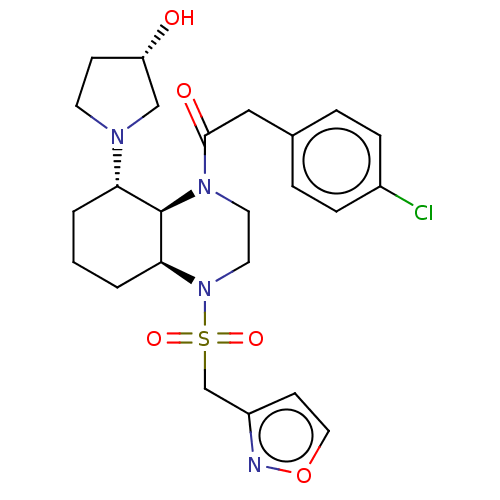 (CHEMBL4096405) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138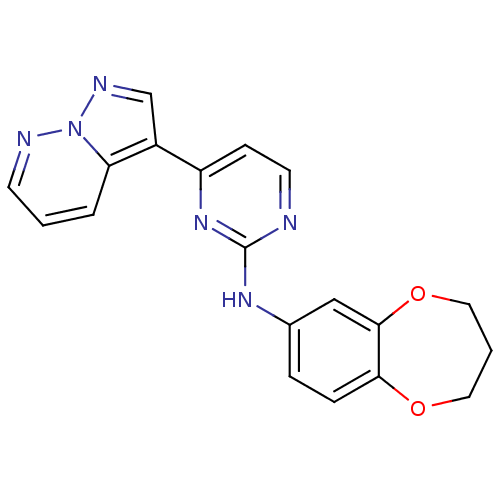 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236876 (CHEMBL4070248) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the inhibition of [3H]WIN-35428 binding to Dopamine transporter in rat brain tissue | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236873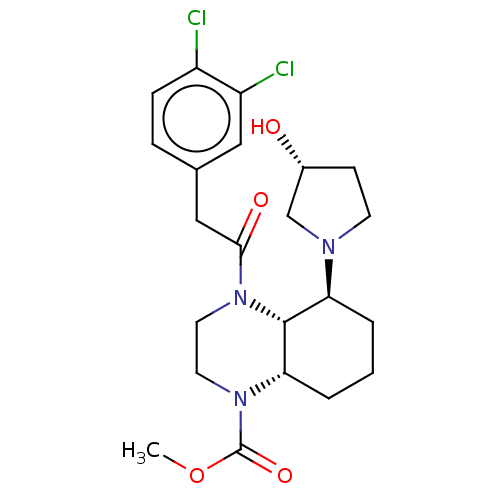 (CHEMBL4099840) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from serotonin transporter of rat brain | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236877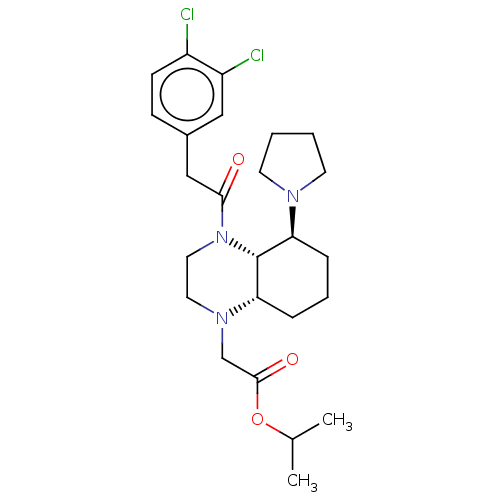 (CHEMBL4064388) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236883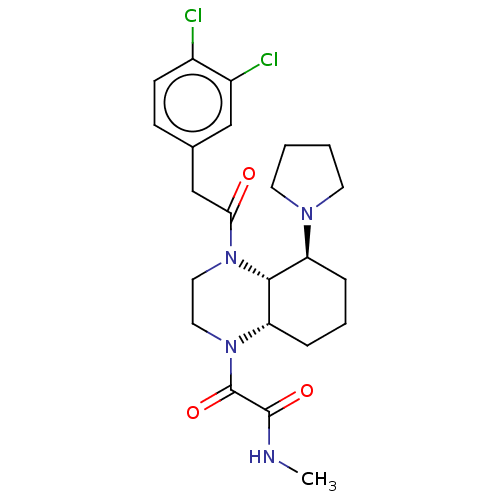 (CHEMBL4092106) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236869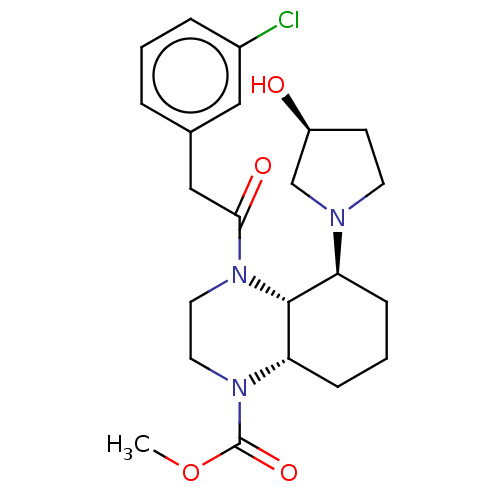 (CHEMBL4092100) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]NE from Norepinephrine transporter of rat brain | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236884 (CHEMBL4060558) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236867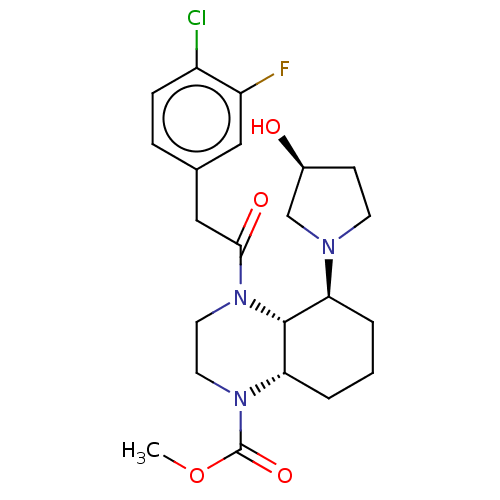 (CHEMBL4083401) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8140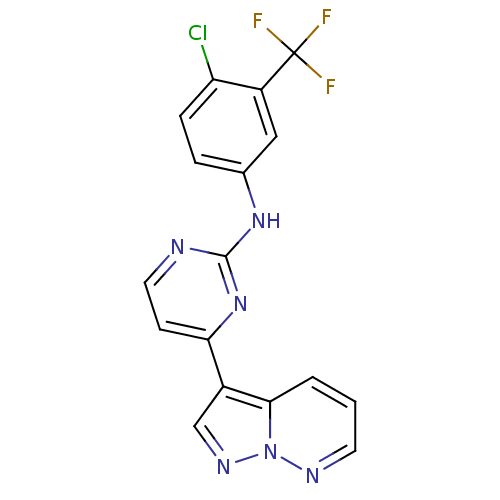 (N-[4-chloro-3-(trifluoromethyl)phenyl]-4-{pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8142 (N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8144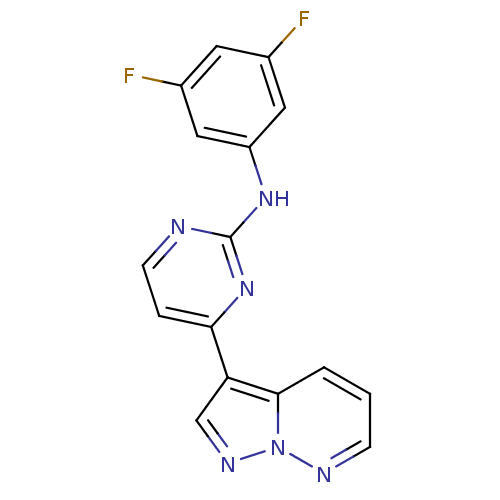 (N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8171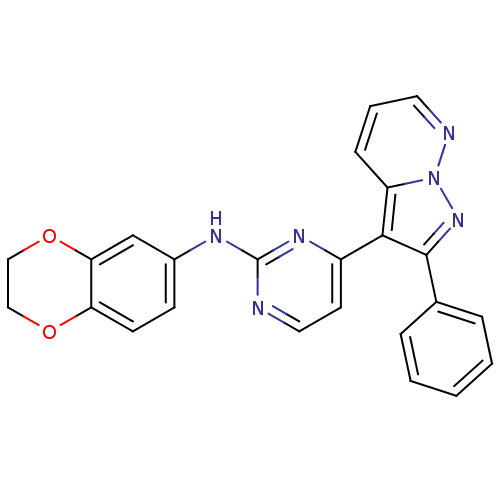 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-phenylpy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8129 (4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8130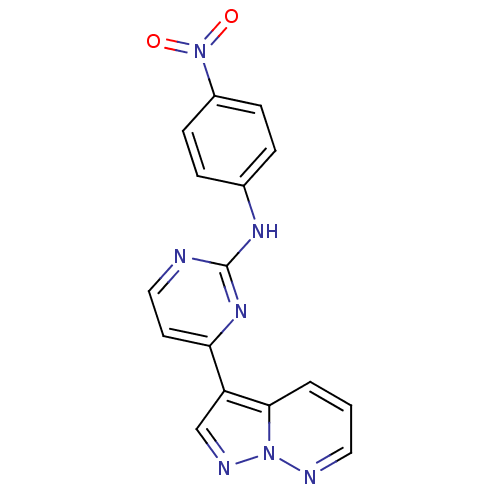 (N-(4-nitrophenyl)-4-{pyrazolo[1,5-a]pyridazin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8135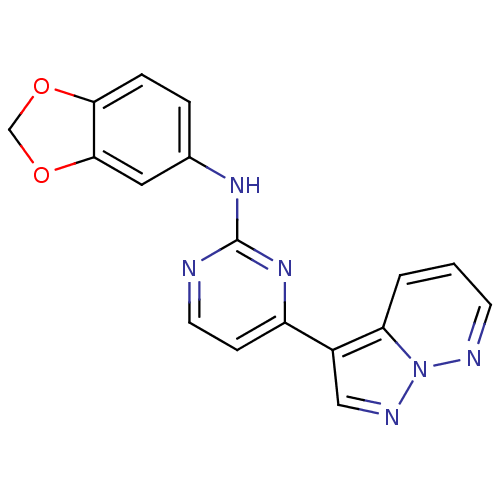 (N-(2H-1,3-benzodioxol-5-yl)-4-{pyrazolo[1,5-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236885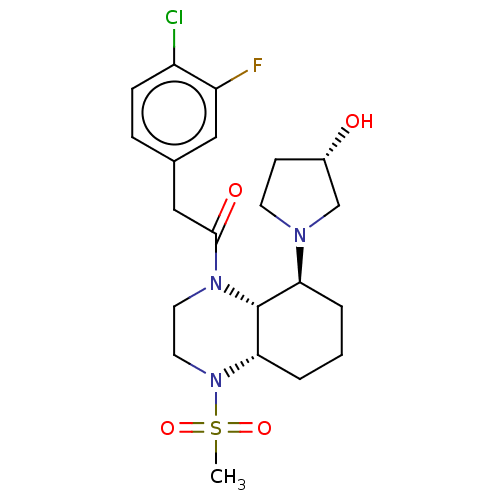 (CHEMBL4088788) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM54795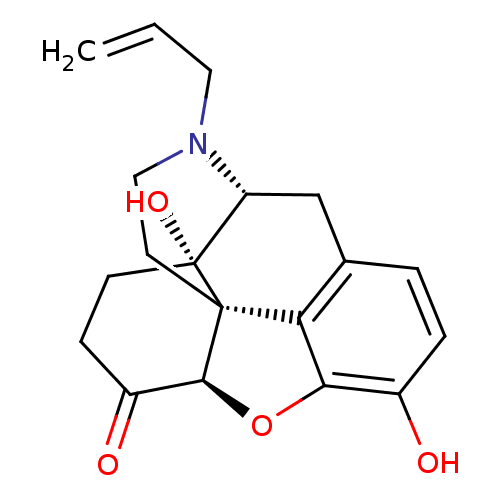 ((4R,4aS,7aR,12bS)-3-allyl-4a,9-dihydroxy-2,4,5,6,7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-type opioid receptor in guinea pig brain membranes after 120 mins by solid scintillation counting | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50236880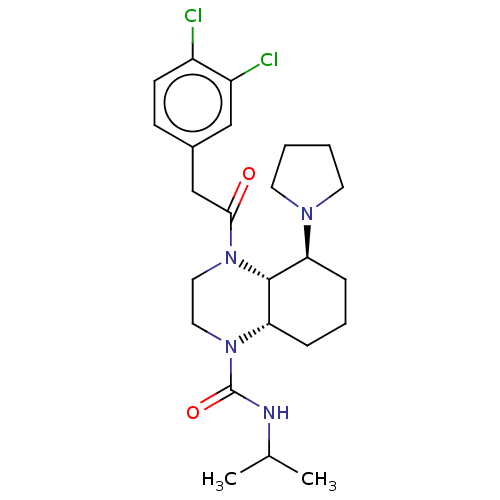 (CHEMBL4102311) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Almond | J Med Chem 60: 2526-2551 (2017) Article DOI: 10.1021/acs.jmedchem.6b01868 BindingDB Entry DOI: 10.7270/Q20C4Z1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386346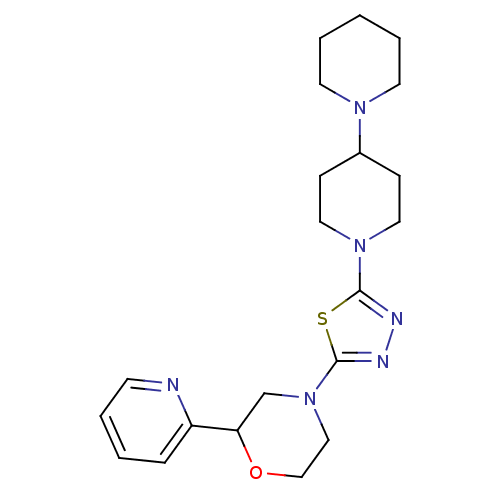 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386351 (CHEMBL2048589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386364 (CHEMBL2048592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora C kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1380 total ) | Next | Last >> |