

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007568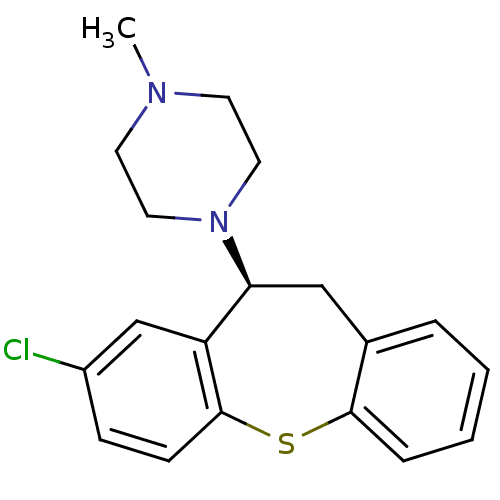 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001786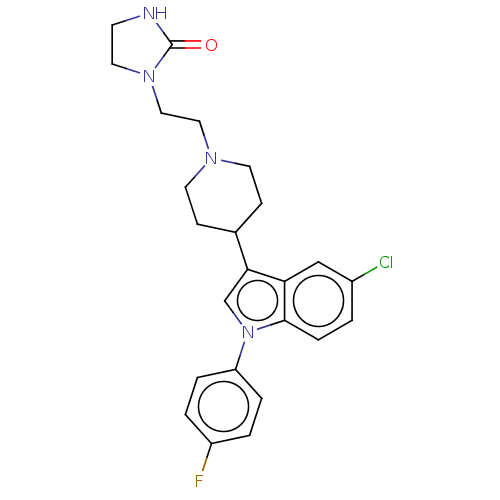 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin (0.5 nM) from rat cerebral cortex 5-hydroxytryptamine 2A receptors | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50007568 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122815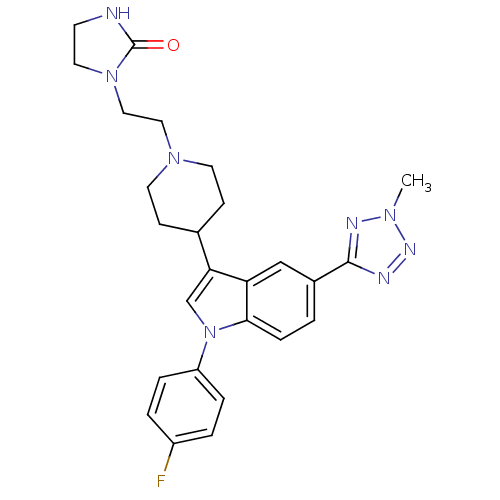 (1-(2-{4-[1-(4-Fluoro-phenyl)-5-(2-methyl-2H-tetraz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50152456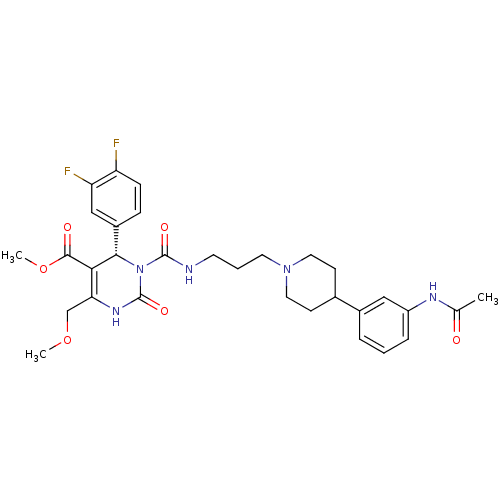 ((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]T226296 from rat recombinant MCH1 receptor | J Med Chem 50: 3883-90 (2007) Article DOI: 10.1021/jm060383x BindingDB Entry DOI: 10.7270/Q25D8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50152456 ((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells | J Med Chem 50: 3870-82 (2007) Article DOI: 10.1021/jm060381c BindingDB Entry DOI: 10.7270/Q2930SWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50001786 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122813 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-(2-methyl-2H-tetraz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50001786 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50122817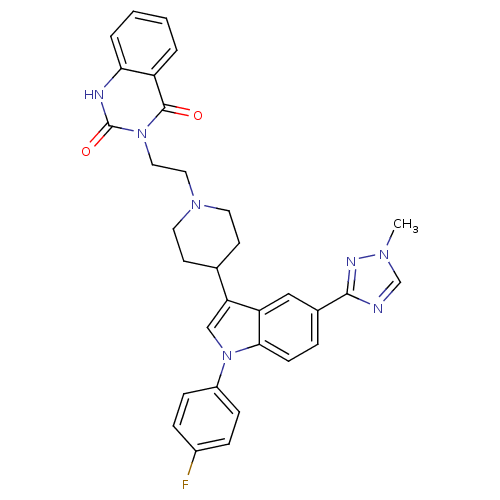 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-[1,2,4...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001786 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]spiperone (0.5 nM) from rat corpus striatum dopamine D2 receptor | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50122826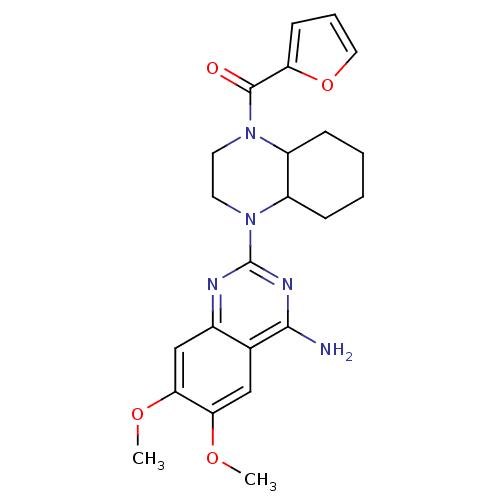 ((Cyclazosin)[4-(4-Amino-6,7-dimethoxy-quinazolin-2...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007567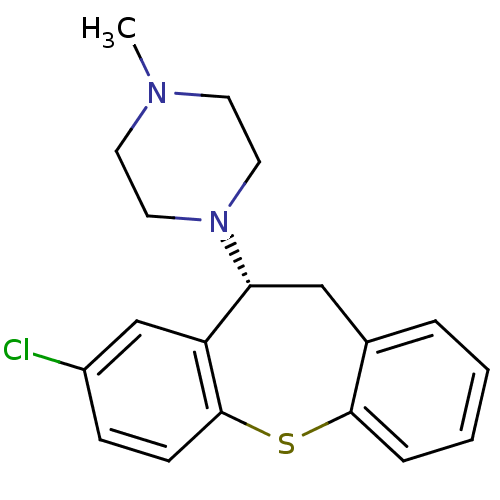 (1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122799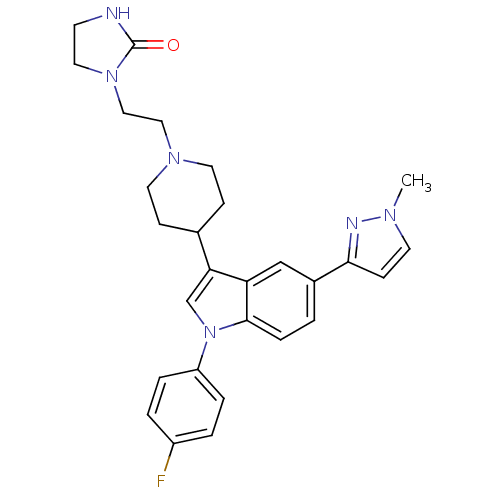 (1-(2-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50001786 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine (0.5 nM) from rat 5-hydroxytryptamine 2C receptor expressed in SR-3T3 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122808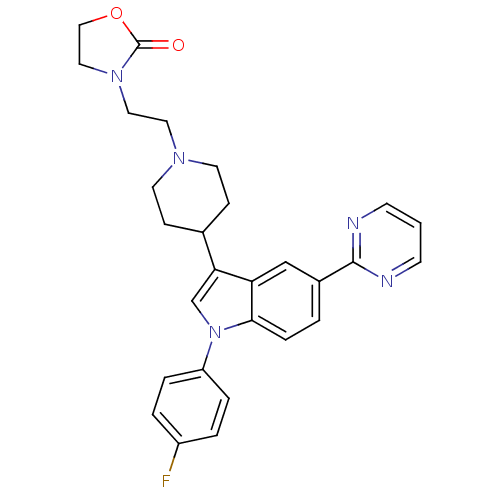 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-pyrimidin-2-yl-1H-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50007568 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50219036 (CHEMBL388440 | N-{3-[1-(3-{[hydroxy(diphenyl)acety...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells | J Med Chem 50: 3870-82 (2007) Article DOI: 10.1021/jm060381c BindingDB Entry DOI: 10.7270/Q2930SWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50353151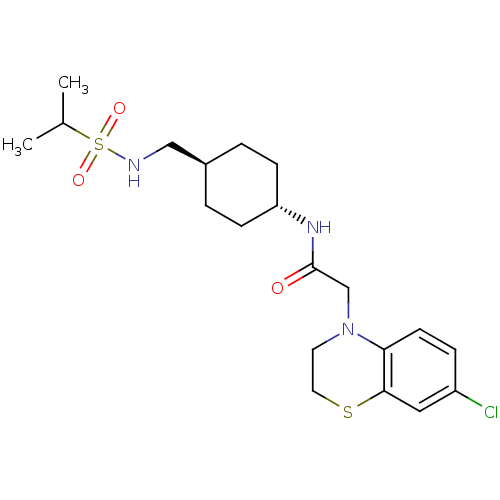 (CHEMBL1829323) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor expressed in thymidine kinase deficient mouse LM cells after 120 mins by scintillation counting | Bioorg Med Chem Lett 21: 5573-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.078 BindingDB Entry DOI: 10.7270/Q2TQ61X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122824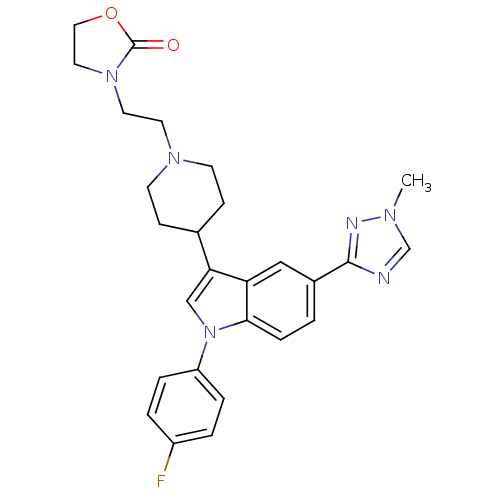 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-[1,2,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50219040 (CHEMBL429464 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells | J Med Chem 50: 3870-82 (2007) Article DOI: 10.1021/jm060381c BindingDB Entry DOI: 10.7270/Q2930SWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50001786 (1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50122813 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-(2-methyl-2H-tetraz...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122811 (1-(2-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-[1,2,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50353150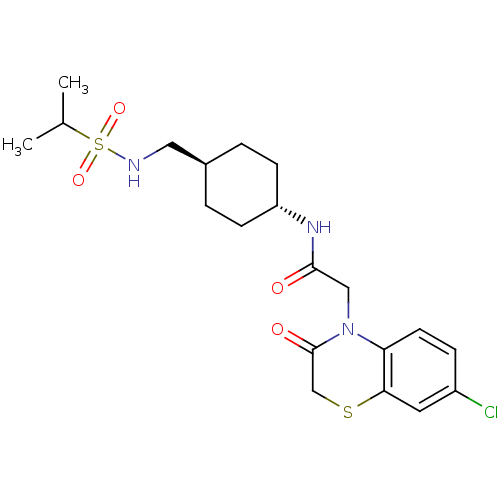 (CHEMBL1829322) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor expressed in thymidine kinase deficient mouse LM cells after 120 mins by scintillation counting | Bioorg Med Chem Lett 21: 5573-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.078 BindingDB Entry DOI: 10.7270/Q2TQ61X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354066 (CHEMBL1836324) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50122826 ((Cyclazosin)[4-(4-Amino-6,7-dimethoxy-quinazolin-2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354068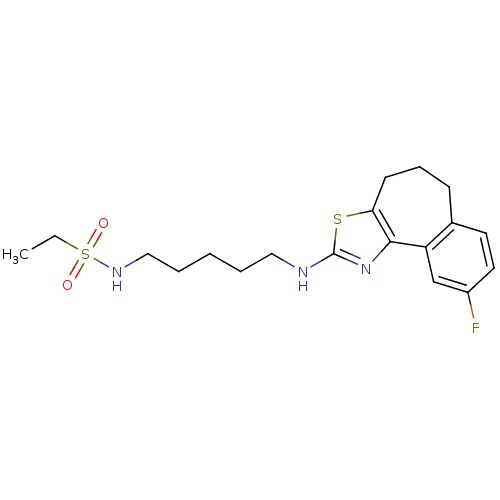 (CHEMBL1836322) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50353154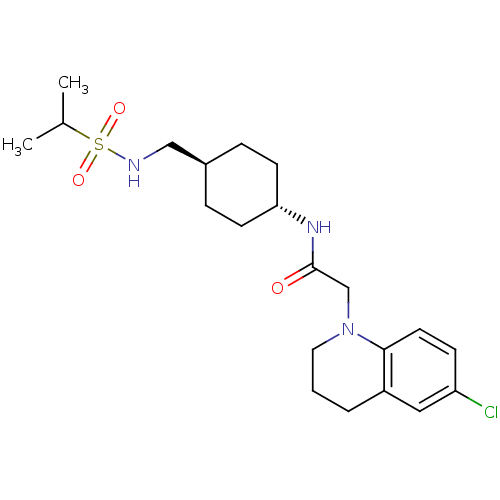 (CHEMBL1829326) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor expressed in thymidine kinase deficient mouse LM cells after 120 mins by scintillation counting | Bioorg Med Chem Lett 21: 5573-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.078 BindingDB Entry DOI: 10.7270/Q2TQ61X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM126004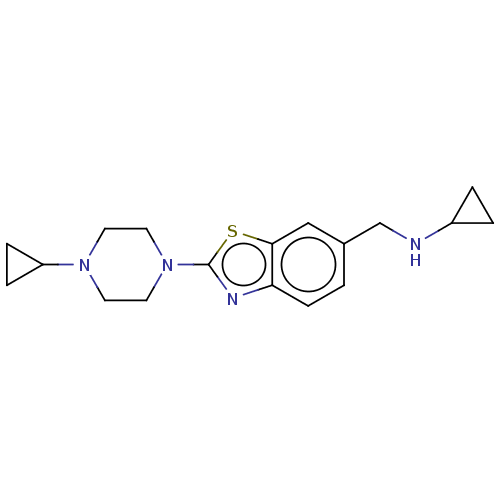 (US8772285, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
High Point Pharmaceuticals, LLC US Patent | Assay Description The ability of the compounds to bind and interact with the human H3 receptor as agonists, inverse agonists and/or antagonists, is determined by a fun... | US Patent US8772285 (2014) BindingDB Entry DOI: 10.7270/Q2P849KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50007567 (1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122810 (3-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-[1,2,4]tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50353148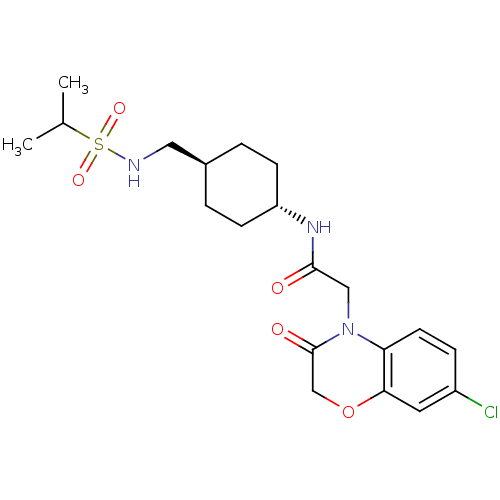 (CHEMBL1829320) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor expressed in thymidine kinase deficient mouse LM cells after 120 mins by scintillation counting | Bioorg Med Chem Lett 21: 5573-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.078 BindingDB Entry DOI: 10.7270/Q2TQ61X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354077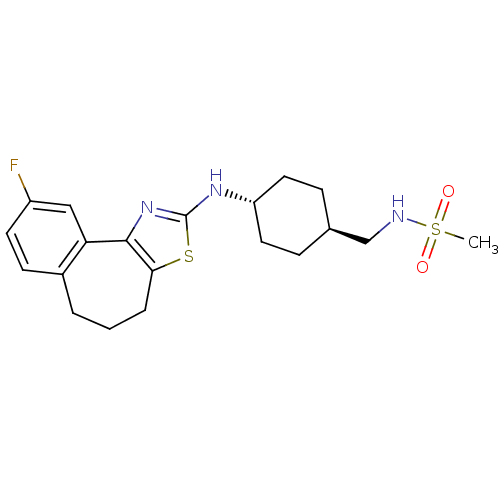 (CHEMBL1836331) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (C.H.O.) | BDBM50122815 (1-(2-{4-[1-(4-Fluoro-phenyl)-5-(2-methyl-2H-tetraz...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.5 nM) from hamster Alpha-1B adrenergic receptor expressed in rat-1 cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354079 (CHEMBL1836329) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50122817 (3-(2-{4-[1-(4-Fluoro-phenyl)-5-(1-methyl-1H-[1,2,4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354070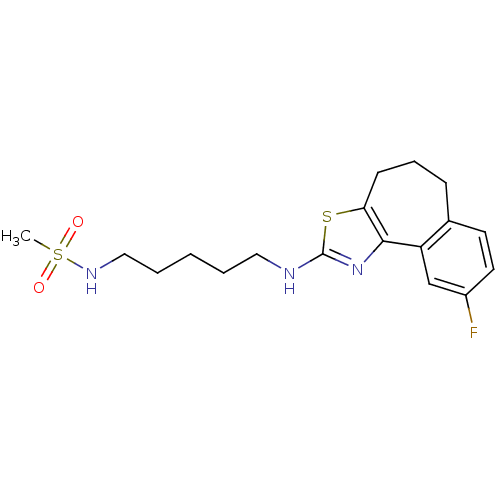 (CHEMBL1836319) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50354072 (CHEMBL1836317) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat NPY5 receptor | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354083 (CHEMBL1836102 | N-{[(1r,4r)-4-{[(4-aminoquinazolin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to NPY5 receptor | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50007567 (1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from bovine Alpha-1A adrenergic receptor expressed in BHK cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50122803 ((SNAP-8719)[4-(4-Amino-6,7-dimethoxy-quinazolin-2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]prazosin (0.3 nM) from rat Alpha-1D adrenergic receptor expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50219026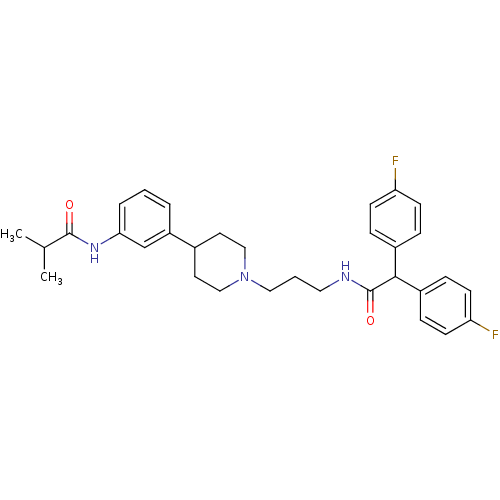 (CHEMBL243338 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells | J Med Chem 50: 3870-82 (2007) Article DOI: 10.1021/jm060381c BindingDB Entry DOI: 10.7270/Q2930SWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354072 (CHEMBL1836317) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50007568 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-YM-09151-2 (0.06 nM) from human Dopamine receptor D4 expressed in CHO cells | J Med Chem 46: 265-83 (2003) Article DOI: 10.1021/jm020938y BindingDB Entry DOI: 10.7270/Q2TB17MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50354078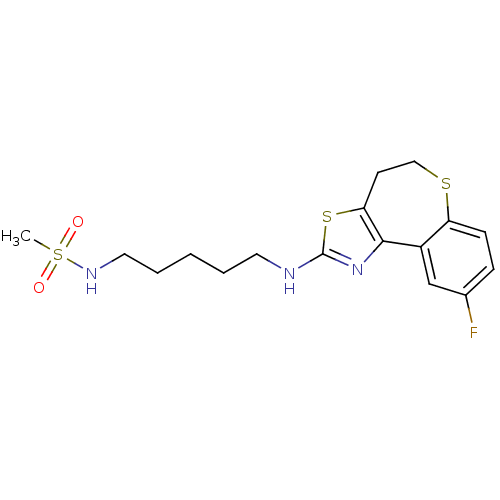 (CHEMBL1836330) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50353152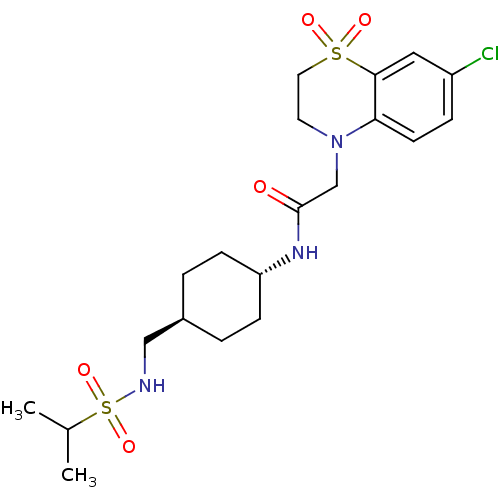 (CHEMBL1829324) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY5 receptor expressed in thymidine kinase deficient mouse LM cells after 120 mins by scintillation counting | Bioorg Med Chem Lett 21: 5573-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.078 BindingDB Entry DOI: 10.7270/Q2TQ61X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1355 total ) | Next | Last >> |