

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347461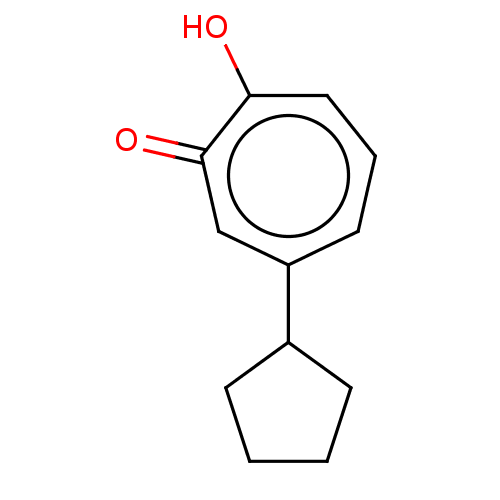 (US9790158, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347454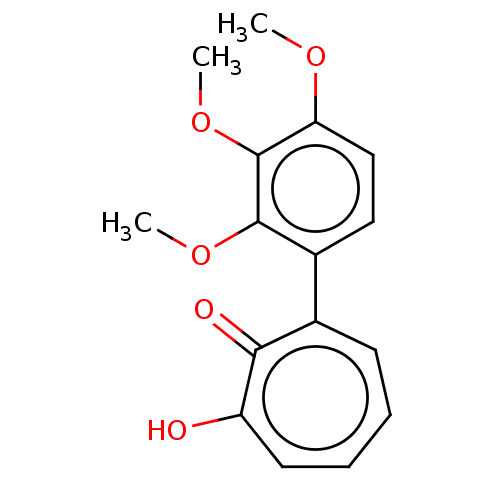 (MO-OH-TM | US9790158, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50492541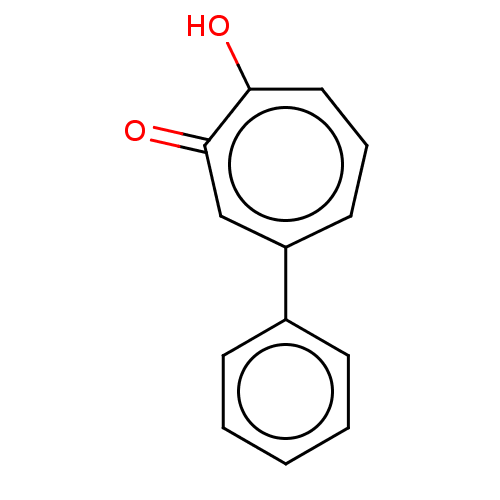 (CHEMBL2408242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347330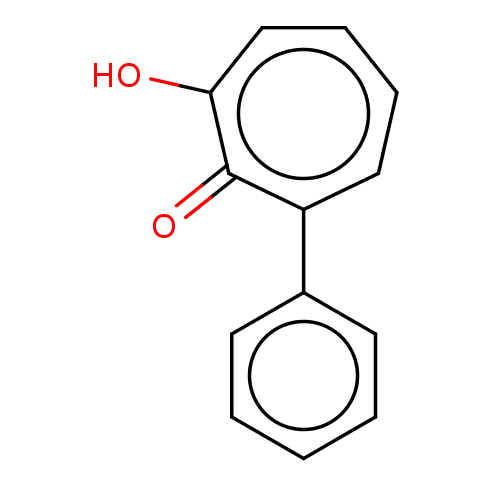 (MO-OH-PHE | US9790158, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50492540 (CHEMBL2408243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM348884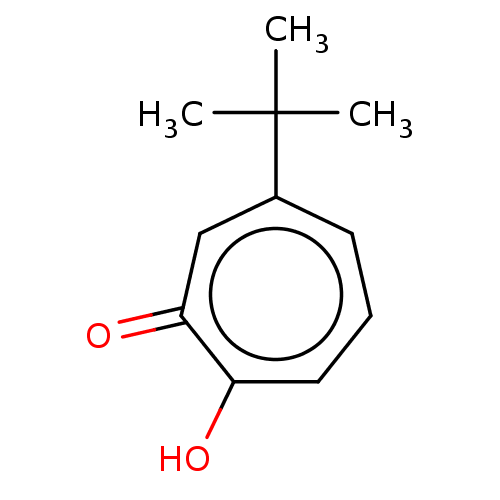 (US9790158, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347452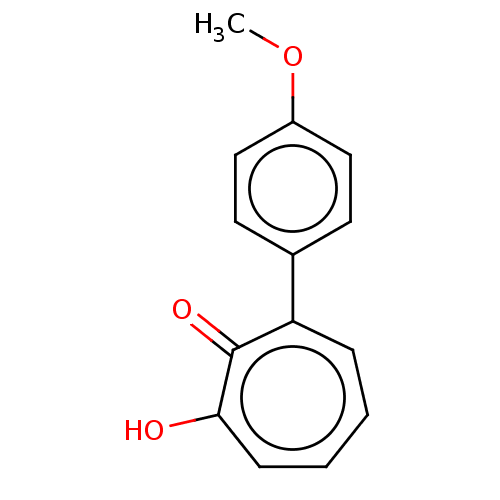 (MO-OH-SM | US9790158, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347460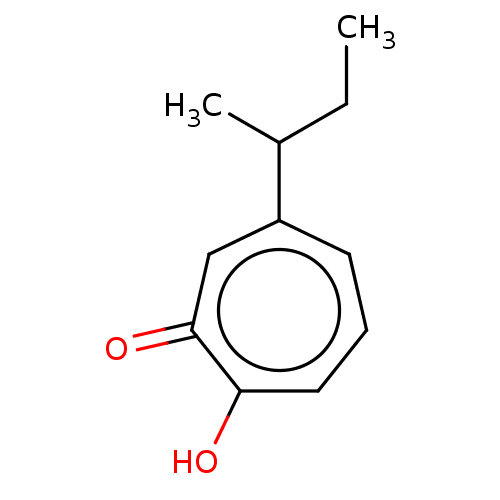 (US9790158, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069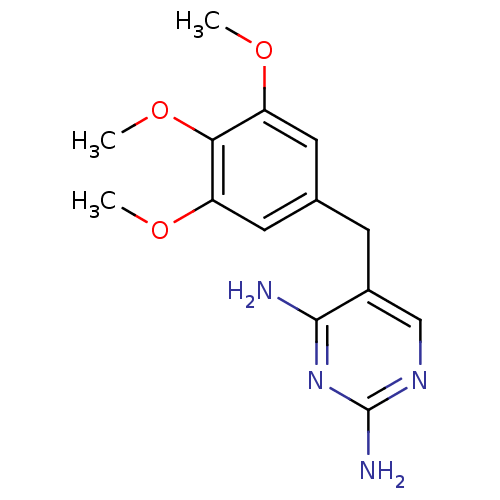 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347454 (MO-OH-TM | US9790158, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347451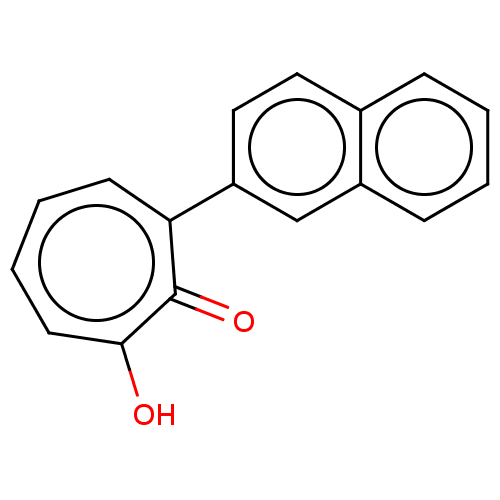 (MO-OH-NAP | US9790158, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347453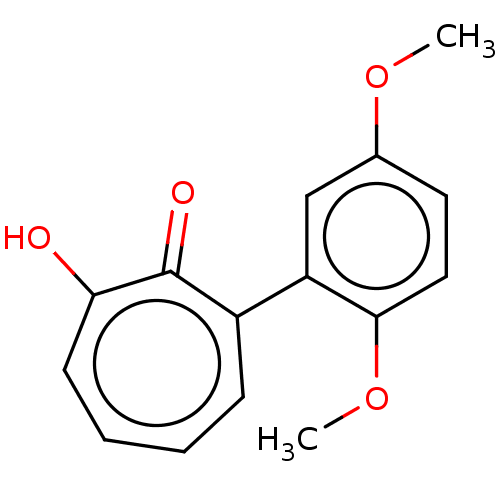 (MO-OH-DM | US9790158, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347453 (MO-OH-DM | US9790158, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347457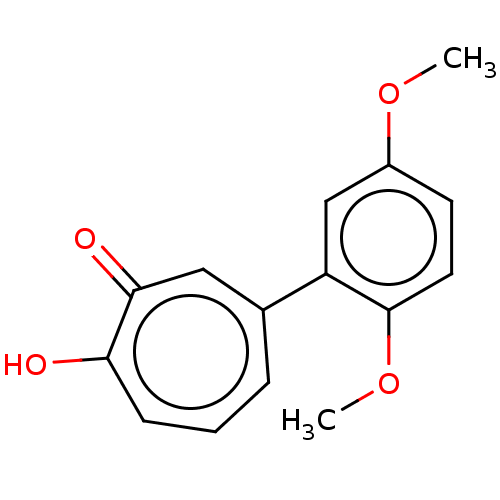 (US9790158, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347451 (MO-OH-NAP | US9790158, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347452 (MO-OH-SM | US9790158, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210930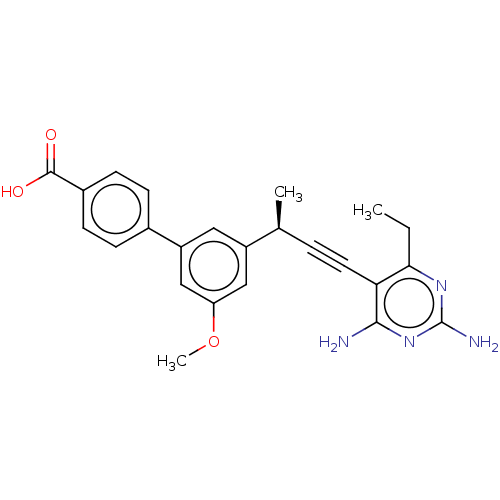 (UCP1173) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347456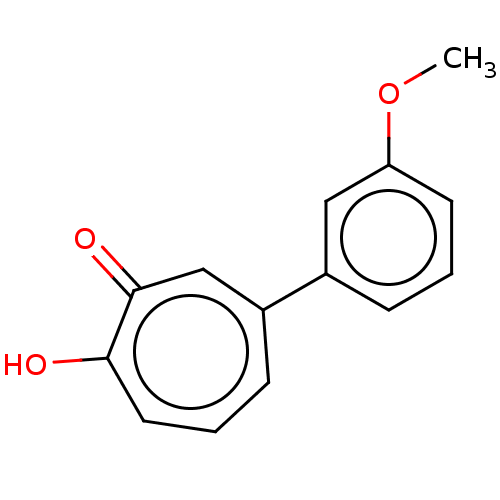 (US9790158, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347457 (US9790158, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50492541 (CHEMBL2408242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50536185 (CHEMBL4588910 | US10870625, Compound 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347456 (US9790158, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210929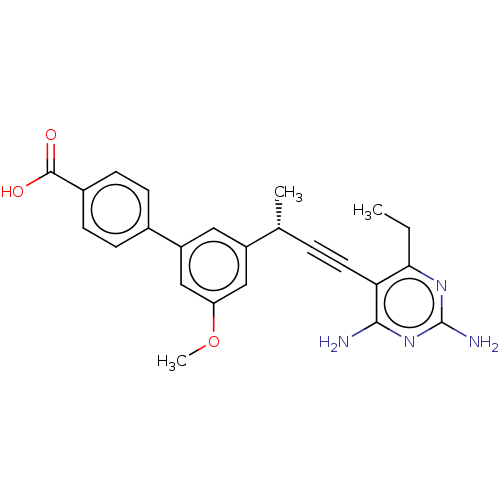 (UCP1172) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190622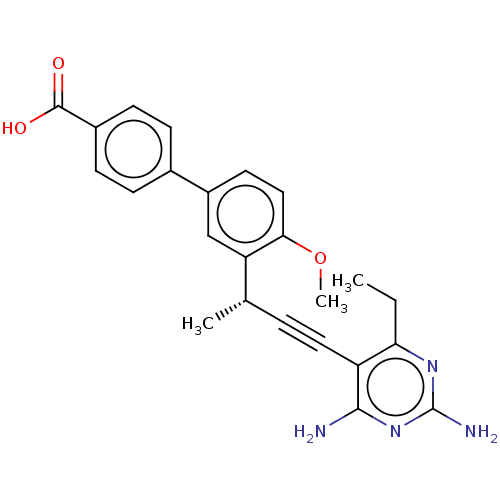 (CHEMBL3827326) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210927 (UCP1039) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347460 (US9790158, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50492540 (CHEMBL2408243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50536188 (CHEMBL4532935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM348884 (US9790158, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50536186 (CHEMBL4483572) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347452 (MO-OH-SM | US9790158, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 670 total ) | Next | Last >> |