 Found 438 hits with Last Name = 'anderson' and Initial = 'ed'
Found 438 hits with Last Name = 'anderson' and Initial = 'ed' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-arginine deiminase type-2
(Homo sapiens (Human)) | BDBM50447757
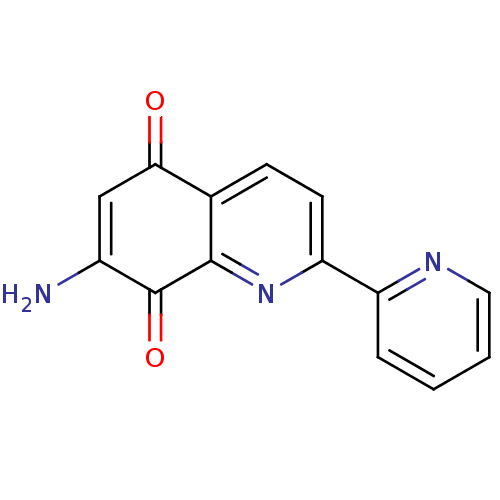
(CHEMBL57818)Show InChI InChI=1S/C14H9N3O2/c15-9-7-12(18)8-4-5-11(17-13(8)14(9)19)10-3-1-2-6-16-10/h1-7H,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD2 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 10 mins followed... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-4
(Homo sapiens (Human)) | BDBM50447757

(CHEMBL57818)Show InChI InChI=1S/C14H9N3O2/c15-9-7-12(18)8-4-5-11(17-13(8)14(9)19)10-3-1-2-6-16-10/h1-7H,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD4 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 10 mins followed... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-1
(Homo sapiens (Human)) | BDBM50447760
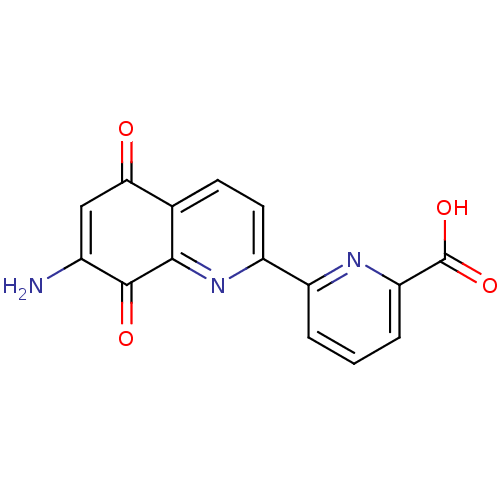
(CHEMBL58150)Show SMILES NC1=CC(=O)c2ccc(nc2C1=O)-c1cccc(n1)C(O)=O |t:1| Show InChI InChI=1S/C15H9N3O4/c16-8-6-12(19)7-4-5-10(18-13(7)14(8)20)9-2-1-3-11(17-9)15(21)22/h1-6H,16H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD1 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-3
(Homo sapiens (Human)) | BDBM50447757

(CHEMBL57818)Show InChI InChI=1S/C14H9N3O2/c15-9-7-12(18)8-4-5-11(17-13(8)14(9)19)10-3-1-2-6-16-10/h1-7H,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD3 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-3
(Homo sapiens (Human)) | BDBM50447761
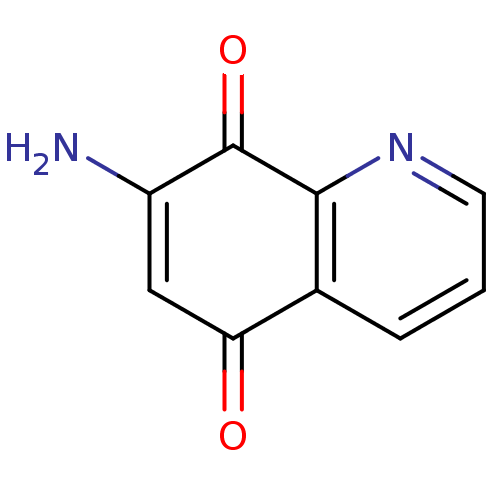
(CHEMBL60908)Show InChI InChI=1S/C9H6N2O2/c10-6-4-7(12)5-2-1-3-11-8(5)9(6)13/h1-4H,10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD3 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-2
(Homo sapiens (Human)) | BDBM50447760

(CHEMBL58150)Show SMILES NC1=CC(=O)c2ccc(nc2C1=O)-c1cccc(n1)C(O)=O |t:1| Show InChI InChI=1S/C15H9N3O4/c16-8-6-12(19)7-4-5-10(18-13(7)14(8)20)9-2-1-3-11(17-9)15(21)22/h1-6H,16H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD2 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 15 mins followed... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-4
(Homo sapiens (Human)) | BDBM50447760

(CHEMBL58150)Show SMILES NC1=CC(=O)c2ccc(nc2C1=O)-c1cccc(n1)C(O)=O |t:1| Show InChI InChI=1S/C15H9N3O4/c16-8-6-12(19)7-4-5-10(18-13(7)14(8)20)9-2-1-3-11(17-9)15(21)22/h1-6H,16H2,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD4 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 15 mins followed... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-2
(Homo sapiens (Human)) | BDBM50447757

(CHEMBL57818)Show InChI InChI=1S/C14H9N3O2/c15-9-7-12(18)8-4-5-11(17-13(8)14(9)19)10-3-1-2-6-16-10/h1-7H,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD2 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 15 mins followed... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-3
(Homo sapiens (Human)) | BDBM50447759
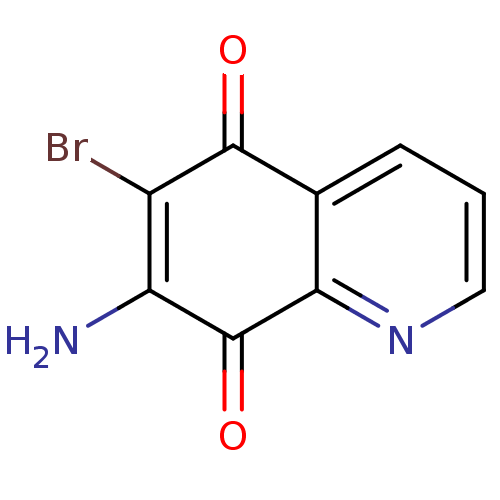
(CHEMBL3113470)Show InChI InChI=1S/C9H5BrN2O2/c10-5-6(11)9(14)7-4(8(5)13)2-1-3-12-7/h1-3H,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD3 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-3
(Homo sapiens (Human)) | BDBM50447758

(CHEBI:9287 | NSC-45383 | Nigrin | Rufocromomycin |...)Show SMILES COC1=C(N)C(=O)c2nc(ccc2C1=O)-c1nc(C(O)=O)c(C)c(c1N)-c1ccc(OC)c(OC)c1O |c:2,(25.46,-.06,;26.8,-.83,;28.13,-.06,;28.13,1.48,;26.79,2.25,;29.46,2.25,;29.46,3.79,;30.79,1.49,;32.13,2.25,;33.46,1.48,;33.46,-.05,;32.14,-.83,;30.79,-.06,;29.47,-.83,;29.47,-2.37,;34.79,2.25,;36.13,1.48,;37.47,2.26,;38.8,1.49,;40.13,2.26,;38.81,-.05,;37.46,3.79,;38.79,4.57,;36.13,4.55,;34.79,3.79,;33.46,4.56,;36.13,6.09,;37.46,6.86,;37.46,8.42,;36.12,9.19,;36.12,10.73,;37.45,11.5,;34.8,8.42,;33.46,9.18,;33.46,10.72,;34.79,6.86,;33.46,6.09,)| Show InChI InChI=1S/C25H22N4O8/c1-9-14(10-6-8-13(35-2)23(36-3)20(10)30)15(26)19(29-17(9)25(33)34)12-7-5-11-18(28-12)22(32)16(27)24(37-4)21(11)31/h5-8,30H,26-27H2,1-4H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute-Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant wild-type PAD3 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su... |
Bioorg Med Chem 22: 1362-9 (2014)
Article DOI: 10.1016/j.bmc.2013.12.064
BindingDB Entry DOI: 10.7270/Q2QV3P05 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602696
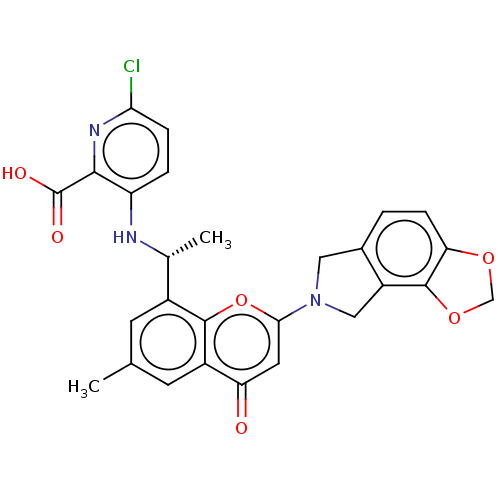
(US11649227, Example 301 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc3OCOc3c2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602696

(US11649227, Example 301 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc3OCOc3c2C1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602697
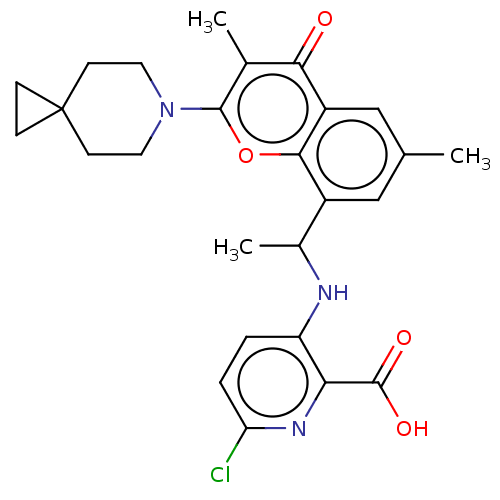
(US11649227, Example 302 | US20230286960, Example 3...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602698

(6-Chloro-3-[1-[3,6-dimethyl-4- oxo-2-(1-piperidyl)...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCCCC1)c(C)c2=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602698

(6-Chloro-3-[1-[3,6-dimethyl-4- oxo-2-(1-piperidyl)...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCCCC1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602699
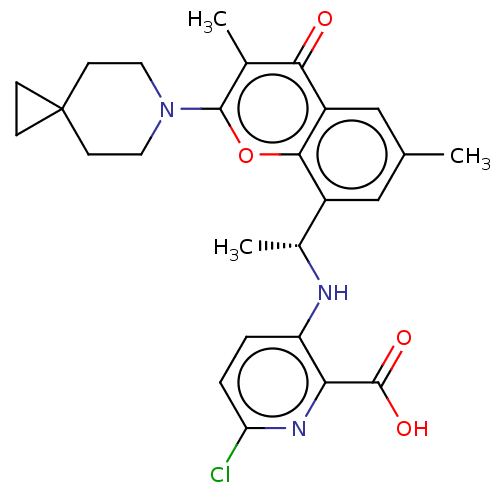
(US11649227, Example 306 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602699

(US11649227, Example 306 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602700
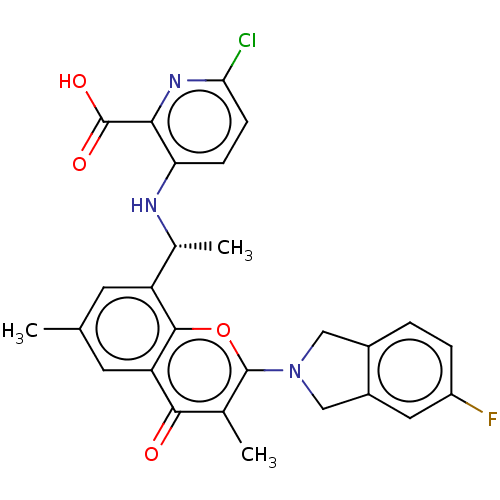
(US11649227, Example 308 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602700

(US11649227, Example 308 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602701

(US11649227, Example 309 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCCCC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602701

(US11649227, Example 309 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCCCC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602702
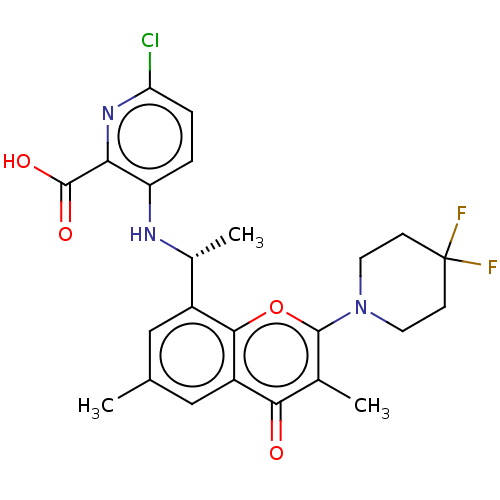
(US11649227, Example 310 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602702

(US11649227, Example 310 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602703

(US11649227, Example 319 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602704
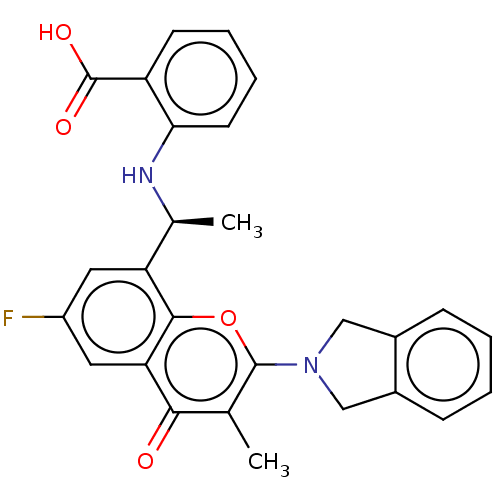
(2-[1-(6-Fluoro-2-isoindolin-2-yl-3-methyl-4-oxo-ch...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602704

(2-[1-(6-Fluoro-2-isoindolin-2-yl-3-methyl-4-oxo-ch...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602705

(2-[1-[2-(4,4-Difluoro-1-piperidyl)-6-fluoro-3-meth...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602705

(2-[1-[2-(4,4-Difluoro-1-piperidyl)-6-fluoro-3-meth...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602706

(2-[1-[2-(6-Azaspiro[2.5]octan-6-yl)-6-fluoro-3-met...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602706

(2-[1-[2-(6-Azaspiro[2.5]octan-6-yl)-6-fluoro-3-met...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602707
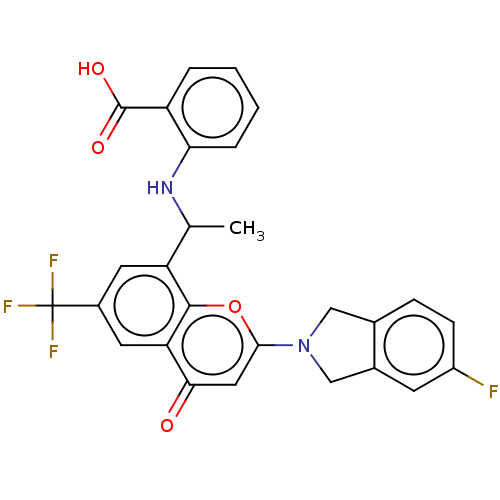
(US11649227, Example 335 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602708

(2-[1-[2-(5-Cyanoisoindolin-2- yl)-4-oxo-6- (triflu...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(cc2C1)C#N)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602708

(2-[1-[2-(5-Cyanoisoindolin-2- yl)-4-oxo-6- (triflu...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(cc2C1)C#N)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602709

(2-[1-[2-Isoindolin-2-yl-4-oxo-6-(trifluoromethyl)c...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccccc2C1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602709

(2-[1-[2-Isoindolin-2-yl-4-oxo-6-(trifluoromethyl)c...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccccc2C1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602710
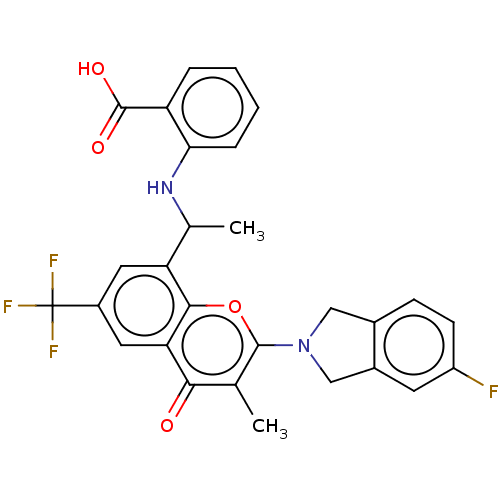
(US11649227, Example 343 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602711

(2-[1-[2-Isoindolin-2-yl-3-methyl-4-oxo-6-(trifluor...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602712
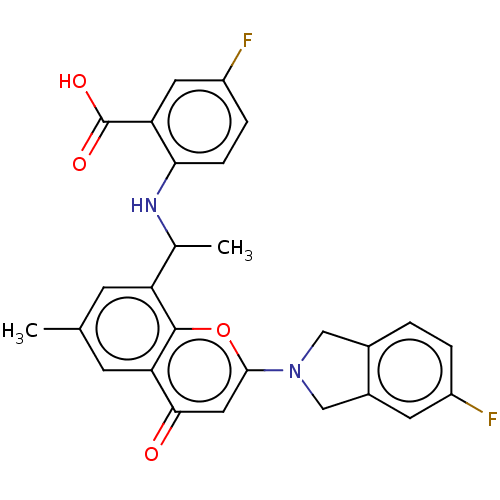
(US11649227, Example 349 | US20230286960, Example 3...)Show SMILES CC(Nc1ccc(F)cc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602712

(US11649227, Example 349 | US20230286960, Example 3...)Show SMILES CC(Nc1ccc(F)cc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602714
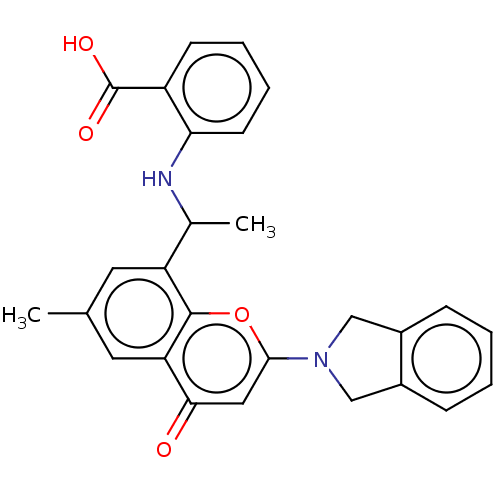
(US11649227, Example 362 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602715
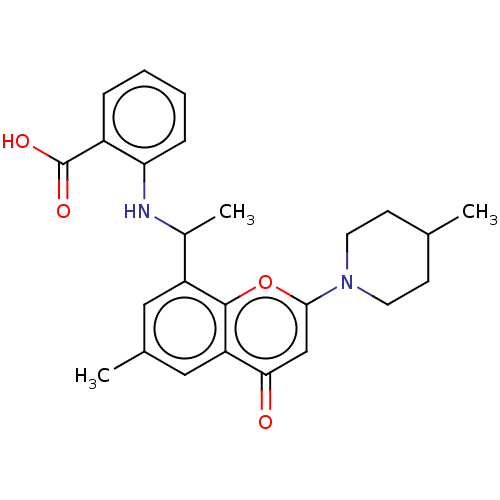
(US11649227, Example 370 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602718
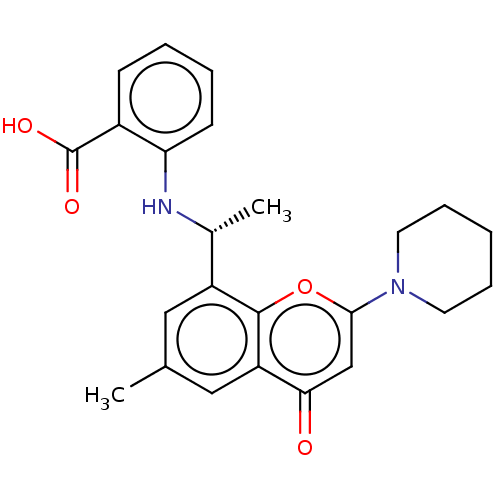
(US11649227, Example 376 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCCCC1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602723

(US11649227, Example 396 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1CCC(F)(F)CC1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602724
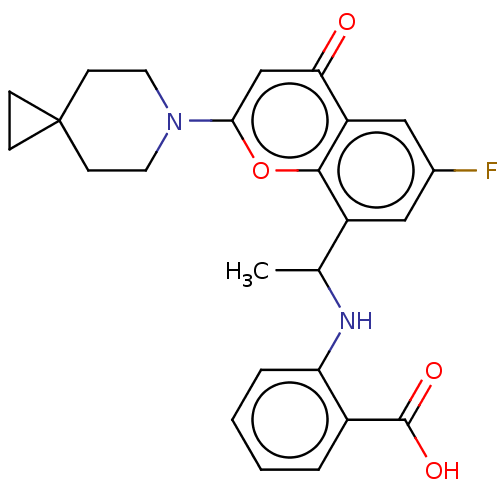
(US11649227, Example 398 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(cc2=O)N1CCC2(CC2)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602725

(US11649227, Example 399 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(cc2=O)N1CCC(F)(F)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602726
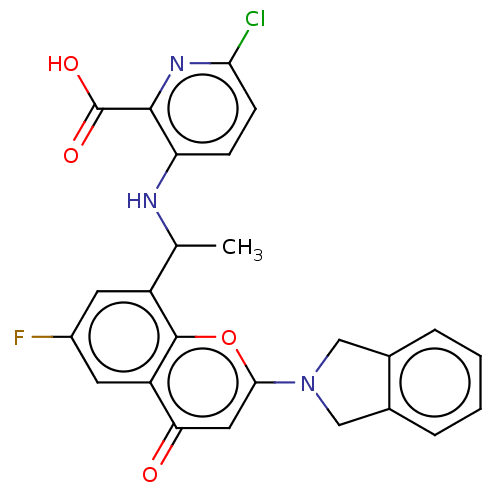
(US11649227, Example 400 | US20230286960, Example 4...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(F)cc2c1oc(cc2=O)N1Cc2ccccc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602726

(US11649227, Example 400 | US20230286960, Example 4...)Show SMILES CC(Nc1ccc(Cl)nc1C(O)=O)c1cc(F)cc2c1oc(cc2=O)N1Cc2ccccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM618133
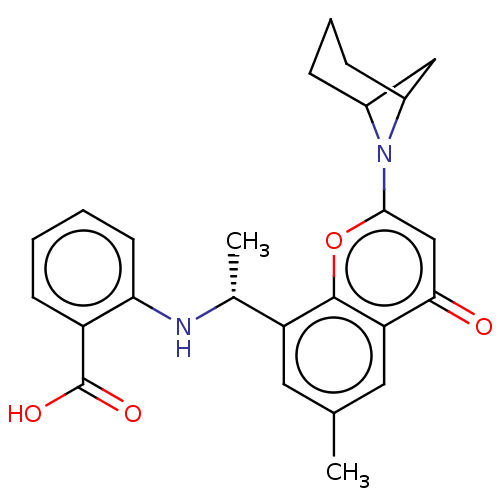
(2-[1-[2-(6-Azabicyclo[3.1.1]heptan-6-yl)-6-methyl-...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1C2CC1CCC2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data