

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483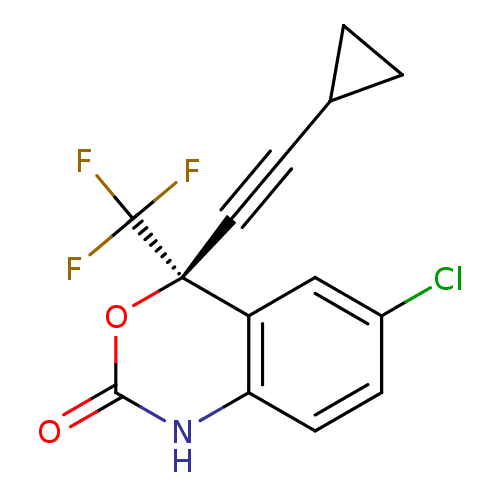 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029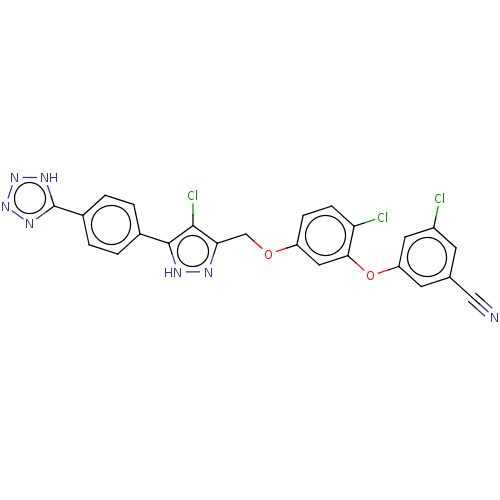 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039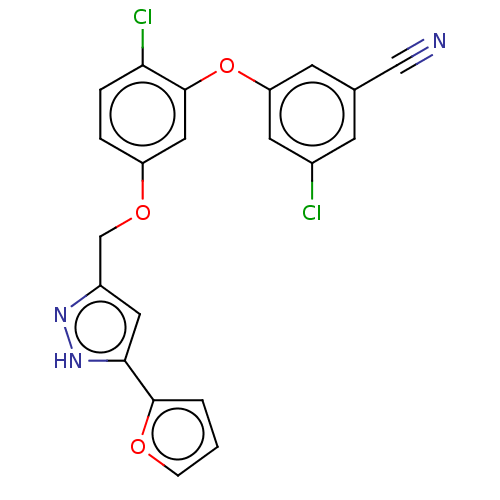 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474799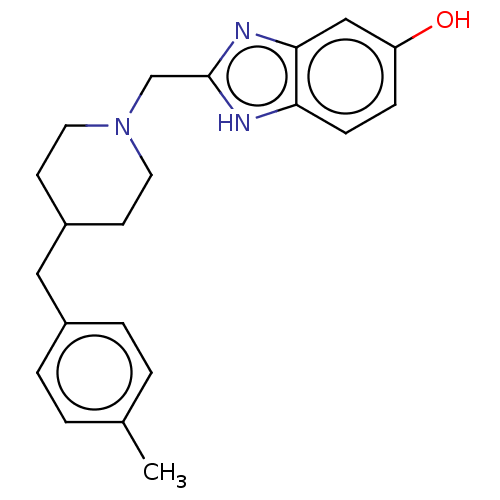 (CHEMBL416690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045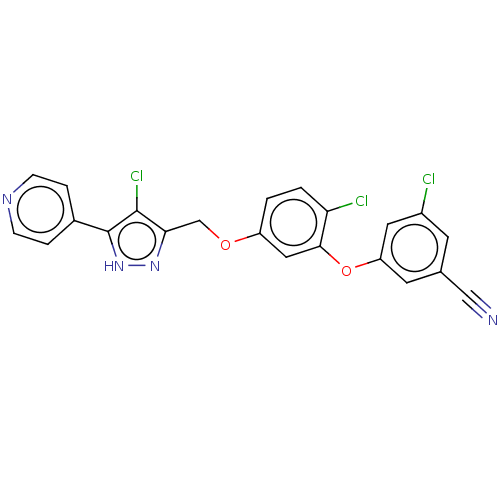 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124914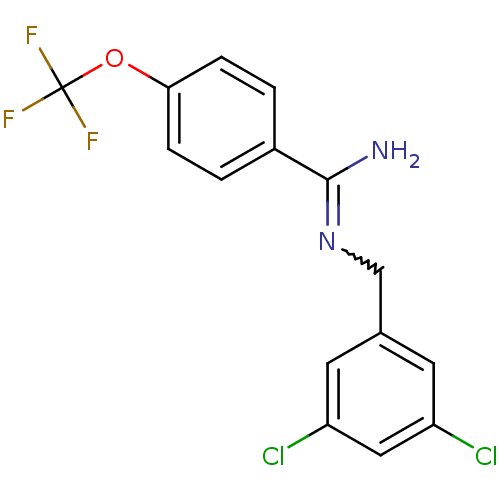 (CHEMBL161180 | N-(3,5-Dichloro-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474791 (CHEMBL65693) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032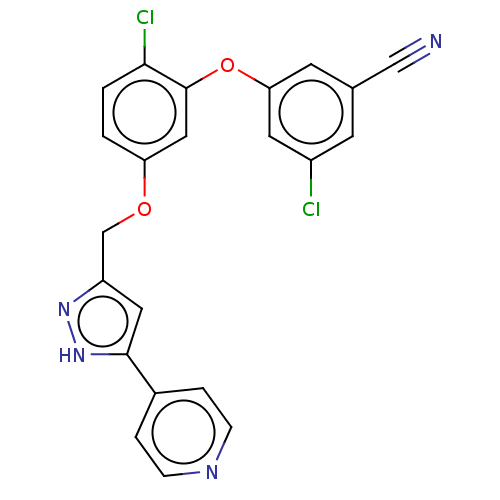 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124885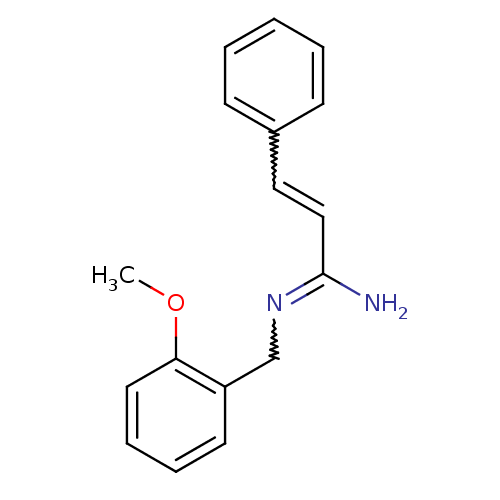 ((E)-N-(2-Methoxy-benzyl)-3-phenyl-acrylamidine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040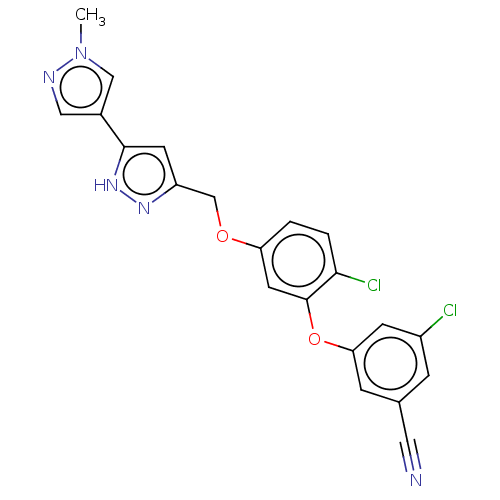 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484031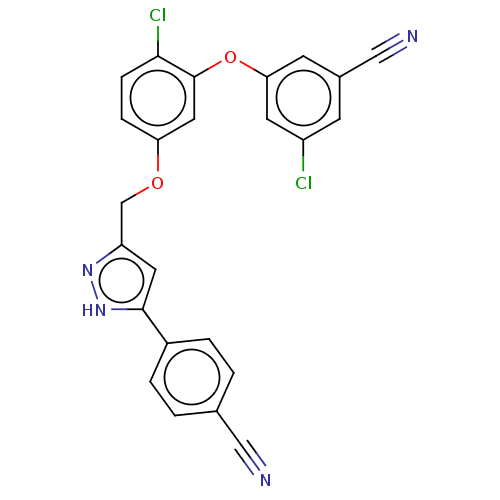 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474809 (CHEMBL65454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474789 (CHEMBL68134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484022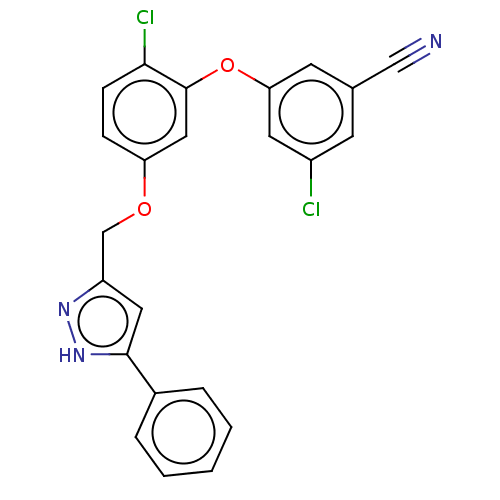 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50204862 (CHEMBL436521 | N-(2-((4-benzylpiperidin-1-yl)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474796 (CHEMBL64941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484023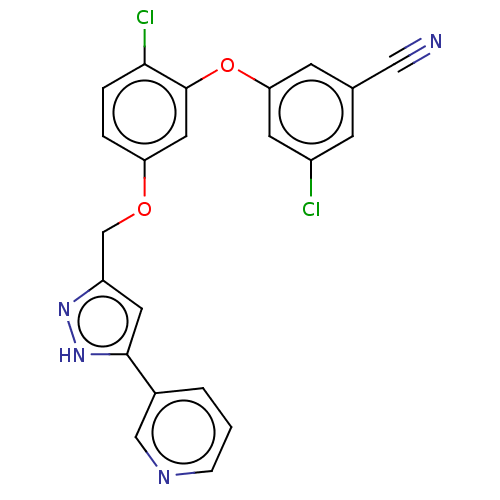 (CHEMBL1801230) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124909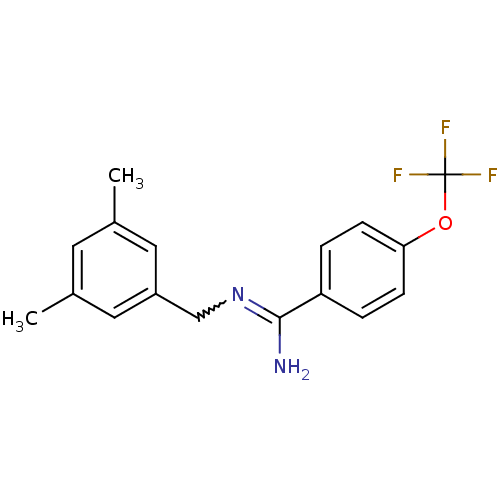 (CHEMBL159744 | N-(3,5-Dimethyl-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50143890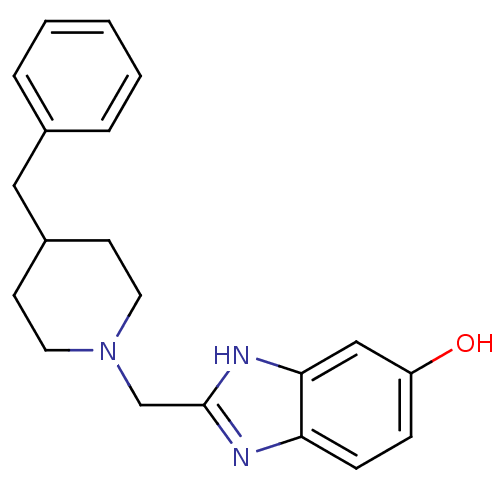 (2-(4-Benzyl-piperidin-1-ylmethyl)-3H-benzoimidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484024 (CHEMBL1801227) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484043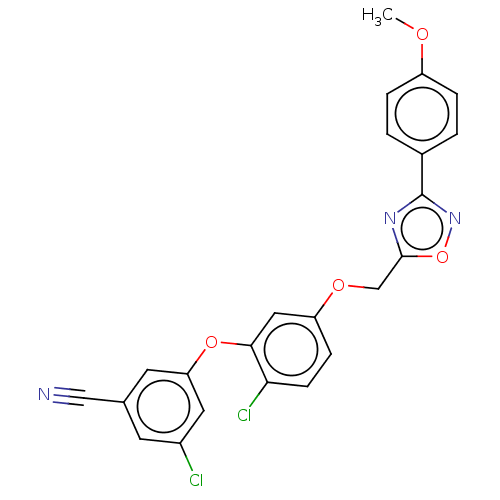 (CHEMBL1801262) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484035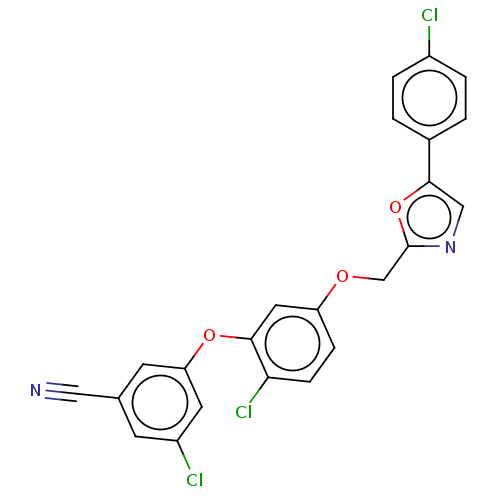 (CHEMBL1801266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484022 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484026 (CHEMBL1801225) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474808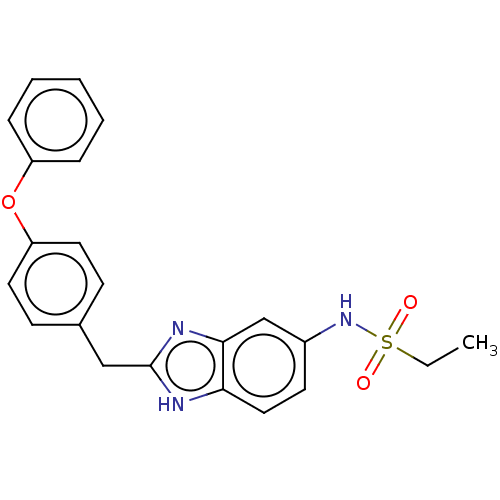 (CHEMBL65314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474792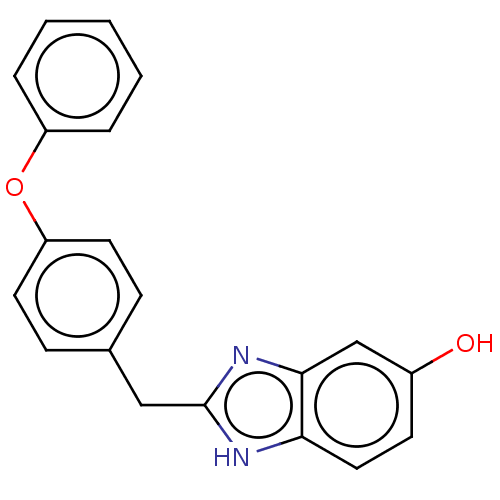 (CHEMBL63200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484033 (CHEMBL1801229) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484031 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124923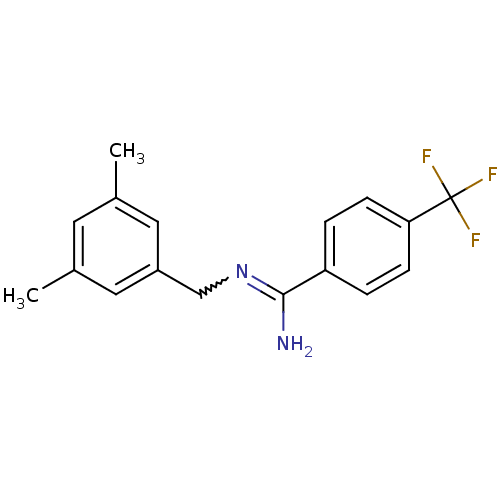 (CHEMBL162080 | N-(3,5-Dimethyl-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484040 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50474790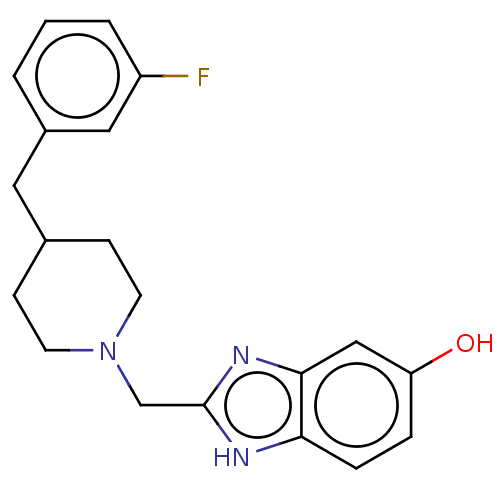 (CHEMBL303832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... | J Med Chem 47: 2089-96 (2004) Article DOI: 10.1021/jm030483s BindingDB Entry DOI: 10.7270/Q2348P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484032 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484035 (CHEMBL1801266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484024 (CHEMBL1801227) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484036 (CHEMBL1801265) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484023 (CHEMBL1801230) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484028 (CHEMBL1801263) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484041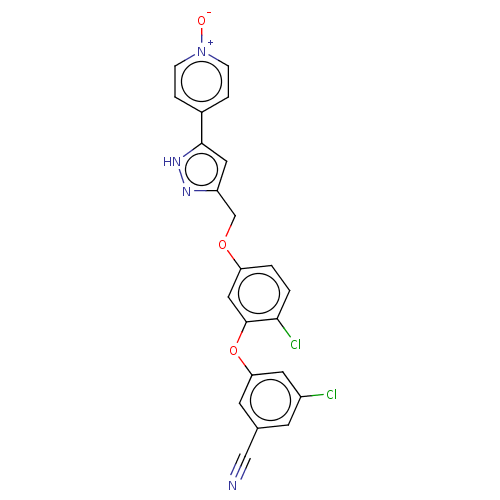 (CHEMBL1801254) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484026 (CHEMBL1801225) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 243 total ) | Next | Last >> |