

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50232153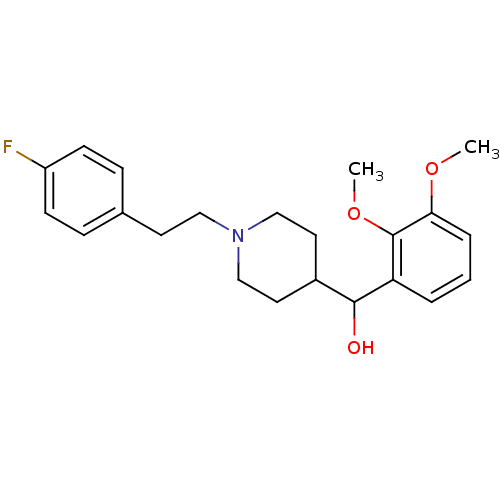 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139371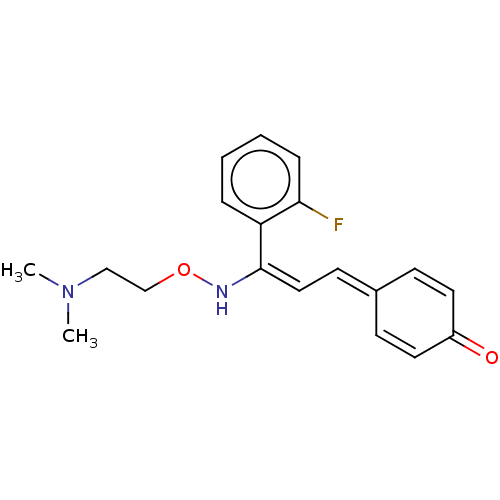 (eplivanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0460 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0830 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.440 | -49.6 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139370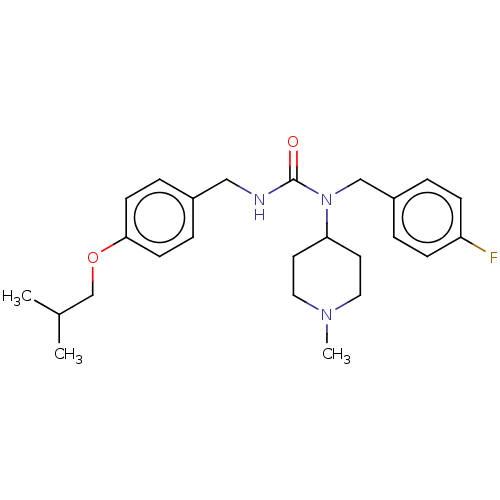 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | -49.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM139371 (eplivanserin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30 | -47.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -47.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM139370 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM86496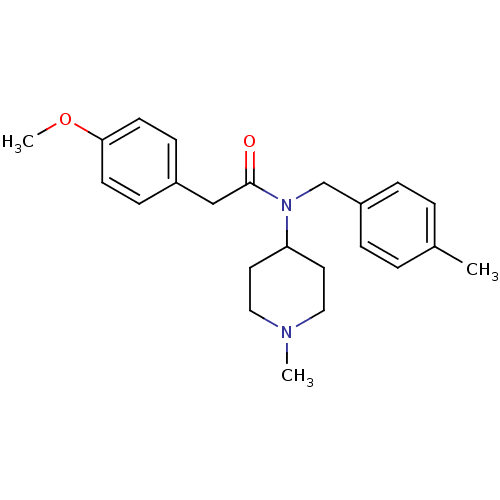 (AC-90179 | N-(4-Methylbenzyl)-N-(1-methyl-4-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50232153 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | -44.7 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc. | Assay Description For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315314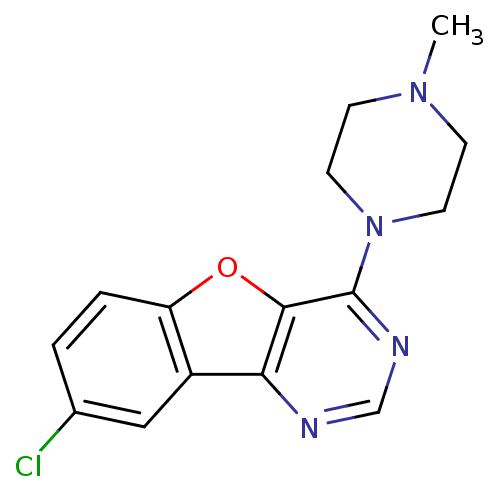 (8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50093777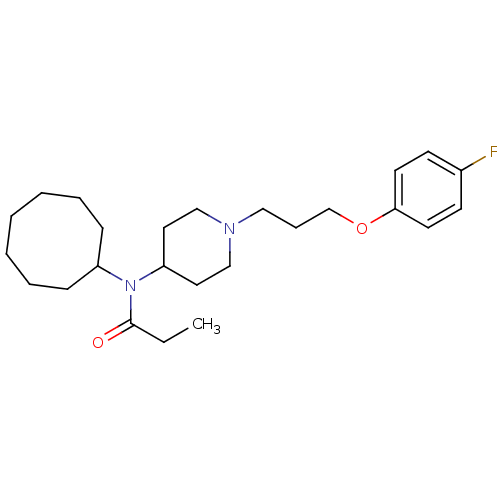 (CHEMBL81377 | N-Cyclooctyl-N-{1-[3-(4-fluoro-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals A/S Curated by ChEMBL | Assay Description Compound was evaluated for its binding against 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 10: 2435-9 (2001) BindingDB Entry DOI: 10.7270/Q2V69HTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 49.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 70.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255598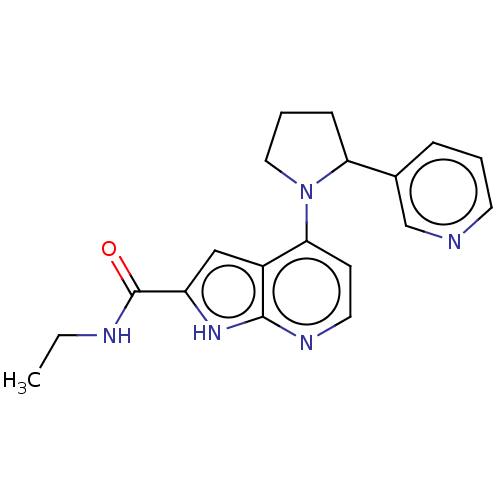 (CHEMBL4064004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255603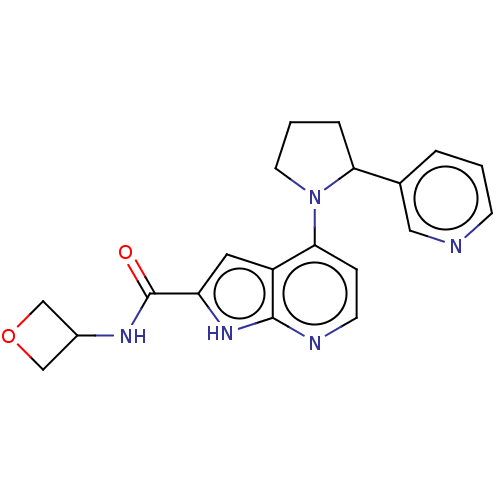 (CHEMBL4083992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255621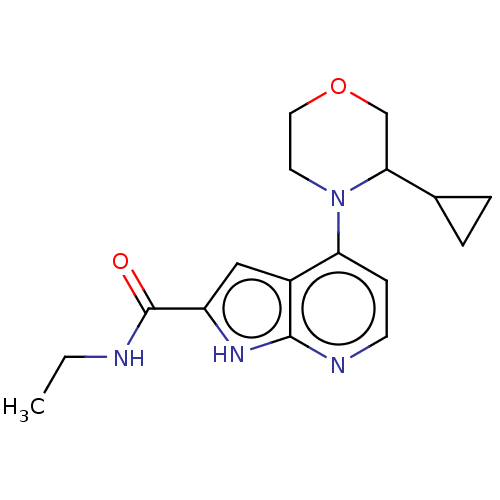 (CHEMBL4070624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255622 (CHEMBL4067896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255581 (CHEMBL4073623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255568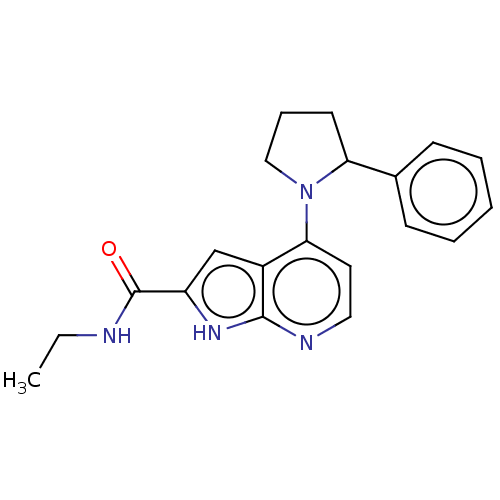 (CHEMBL4091768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255566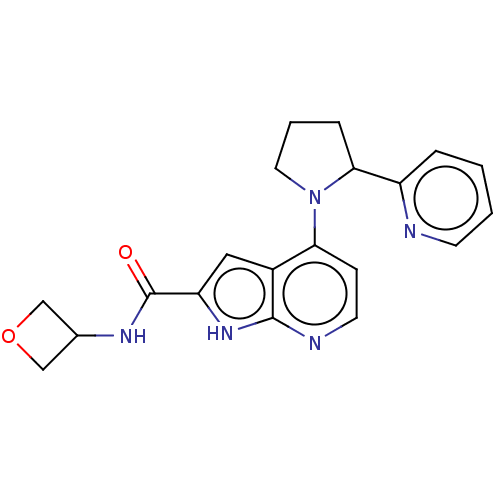 (CHEMBL4094252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255624 (CHEMBL4101983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255582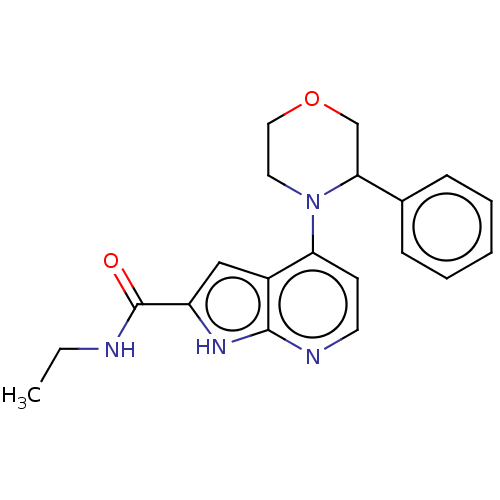 (CHEMBL4078783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM86525 (AMI-193 | CAS_77445 | CHEMBL79834 | NSC_77445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals A/S Curated by ChEMBL | Assay Description Compound was evaluated for its inverse agonist activity against 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 10: 2435-9 (2001) BindingDB Entry DOI: 10.7270/Q2V69HTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583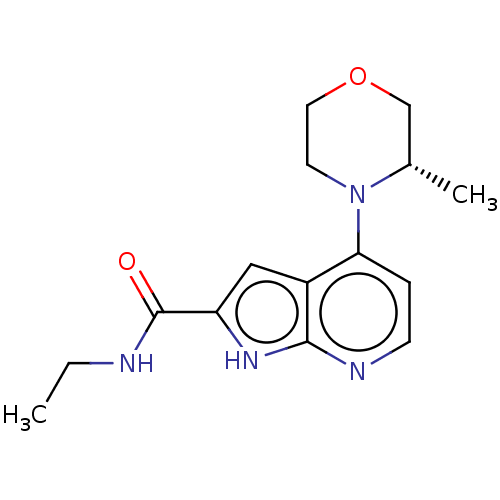 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139371 (eplivanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528011 (US11179399, Example 44_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348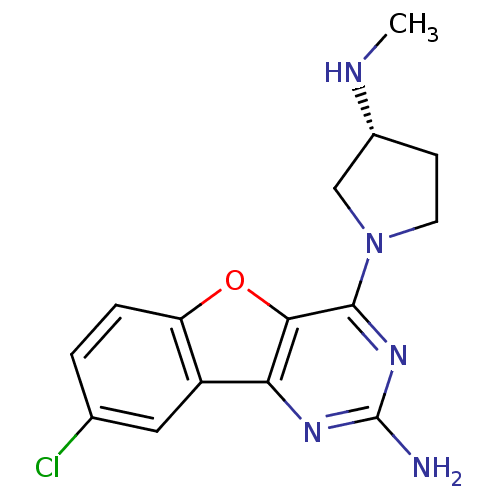 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255623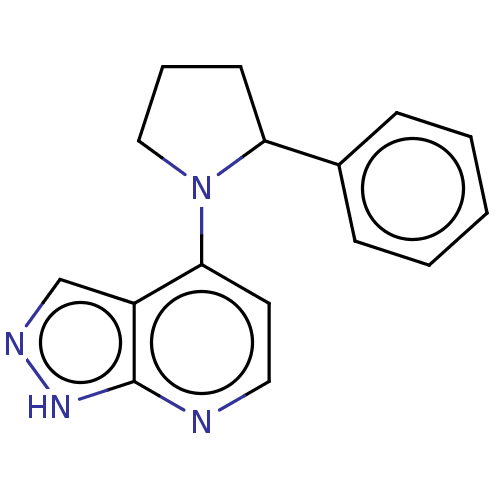 (CHEMBL4072758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50232153 ((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50281169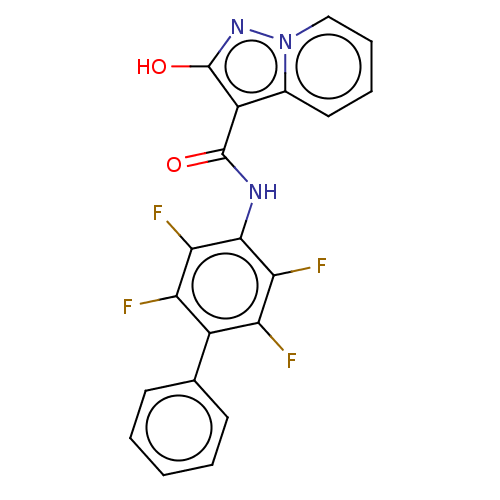 (CHEMBL4173846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human DHODH (31 to 395 residues) expressed in Escherichia coli BL21(DE3) assessed as inhibition of DC... | J Med Chem 61: 6034-6055 (2018) Article DOI: 10.1021/acs.jmedchem.8b00373 BindingDB Entry DOI: 10.7270/Q24X5BBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50108452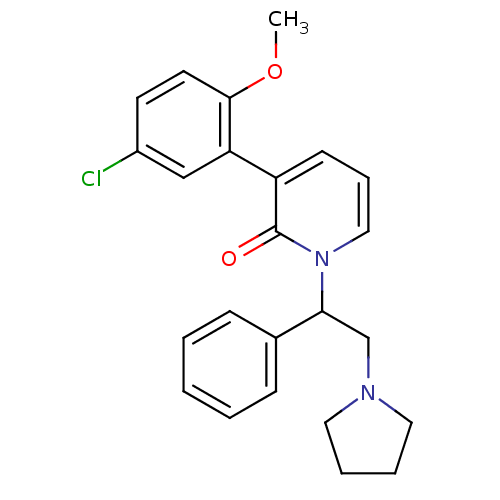 (3-(5-Chloro-2-methoxy-phenyl)-1-(1-phenyl-2-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal Curated by ChEMBL | Assay Description Binding affinity towards kappa opioid receptor by the displacement of [125I]-(D-Pro10)-Dynorphin A | Bioorg Med Chem Lett 12: 197-200 (2001) BindingDB Entry DOI: 10.7270/Q28051XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM15339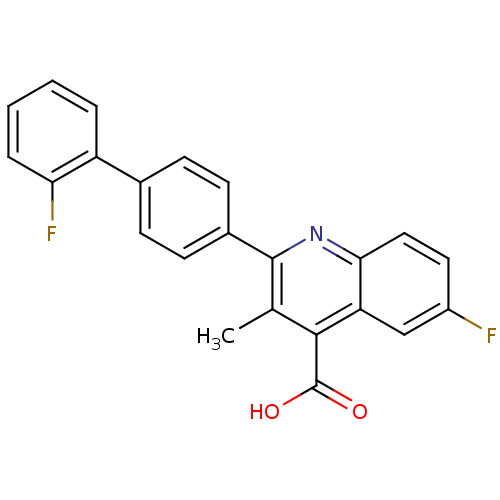 (6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methyl-qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal truncated GST-tagged DHFR (31 to 395 residues) expressed in Escherichia coli BL21(DE3) pyrD using DHO as s... | Eur J Med Chem 129: 287-302 (2017) Article DOI: 10.1016/j.ejmech.2017.02.017 BindingDB Entry DOI: 10.7270/Q2KD215F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM15339 (6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methyl-qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turin Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human DHODH (31 to 395 residues) expressed in Escherichia coli BL21(DE3) assessed as inhibition of DC... | J Med Chem 61: 6034-6055 (2018) Article DOI: 10.1021/acs.jmedchem.8b00373 BindingDB Entry DOI: 10.7270/Q24X5BBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM139370 (ACP-103 | Nuplazid | Pimavanserin | Pimavanserin h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255585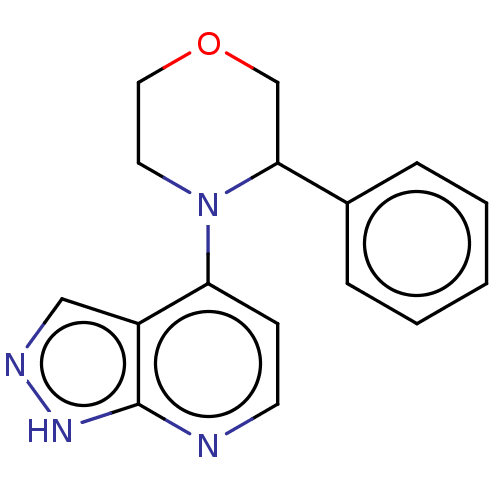 (CHEMBL4094381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130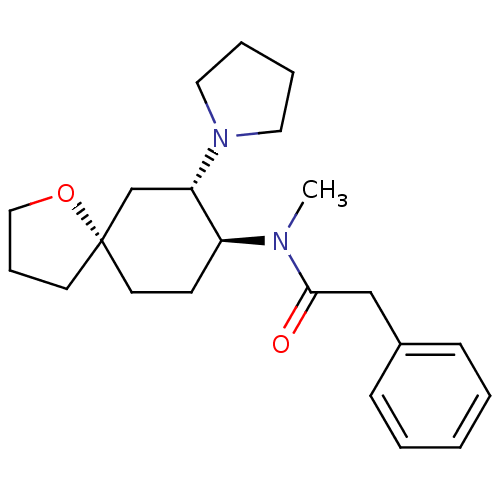 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Binding affinity for 5-HT3 receptor of NG108-15 cells using [3H]-GR-65,630 | Bioorg Med Chem Lett 13: 1141-5 (2003) BindingDB Entry DOI: 10.7270/Q24J0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527982 (US11179399, Example 25_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by the displacement of [125I]-(D-Pro10)-Dynorphin A | Bioorg Med Chem Lett 12: 197-200 (2001) BindingDB Entry DOI: 10.7270/Q28051XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50108437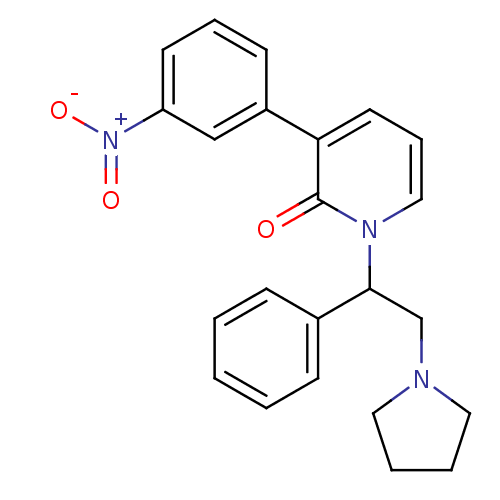 (3-(3-Nitro-phenyl)-1-(1-phenyl-2-pyrrolidin-1-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal Curated by ChEMBL | Assay Description Binding affinity towards kappa opioid receptor by the displacement of [125I]-(D-Pro10)-Dynorphin A | Bioorg Med Chem Lett 12: 197-200 (2001) BindingDB Entry DOI: 10.7270/Q28051XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50108436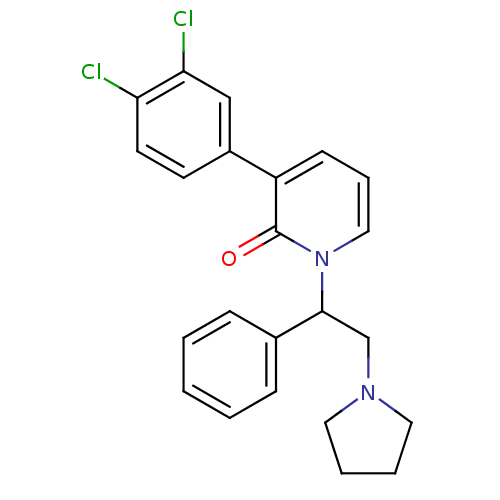 (3-(3,4-Dichloro-phenyl)-1-(1-phenyl-2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal Curated by ChEMBL | Assay Description Binding affinity towards kappa opioid receptor by the displacement of [125I]-(D-Pro10)-Dynorphin A | Bioorg Med Chem Lett 12: 197-200 (2001) BindingDB Entry DOI: 10.7270/Q28051XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50125449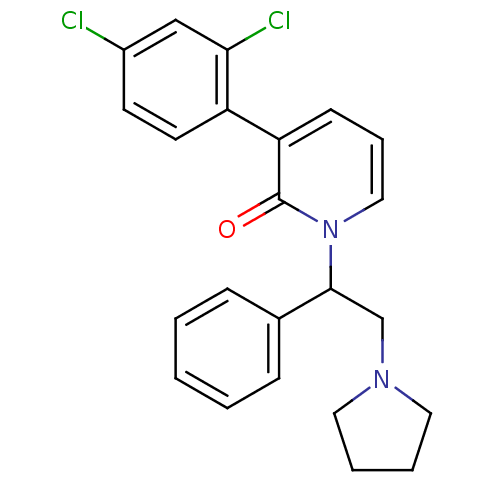 (3-(2,4-Dichloro-phenyl)-1-(1-phenyl-2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Inhibition of [125I]-(D-Pro10)-Dynorphin A binding to human kappa opioid receptor from membranes of HEK293 cells | Bioorg Med Chem Lett 13: 1141-5 (2003) BindingDB Entry DOI: 10.7270/Q24J0DH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50108450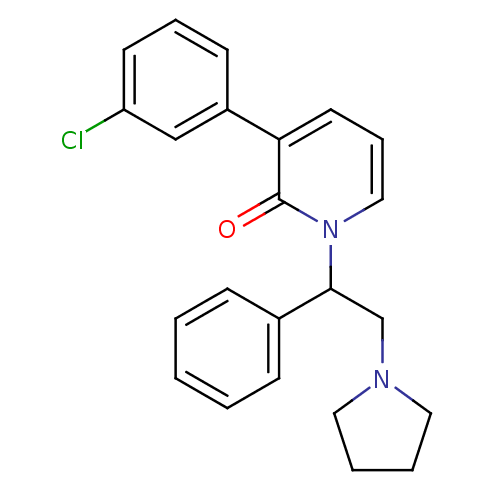 (3-(3-Chloro-phenyl)-1-(1-phenyl-2-pyrrolidin-1-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal Curated by ChEMBL | Assay Description Binding affinity towards kappa opioid receptor by the displacement of [125I]-(D-Pro10)-Dynorphin A | Bioorg Med Chem Lett 12: 197-200 (2001) BindingDB Entry DOI: 10.7270/Q28051XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 501 total ) | Next | Last >> |