 Found 273 hits with Last Name = 'anderton' and Initial = 'm'
Found 273 hits with Last Name = 'anderton' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959
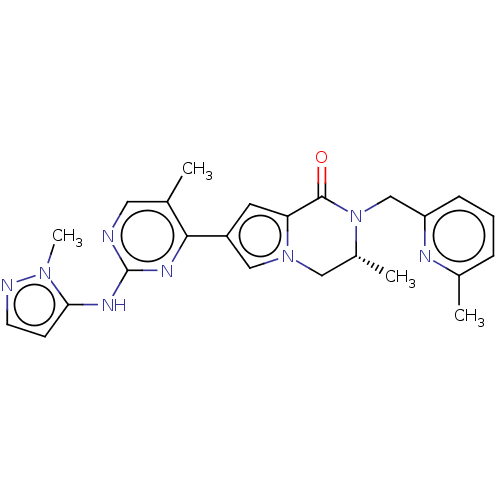
(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988
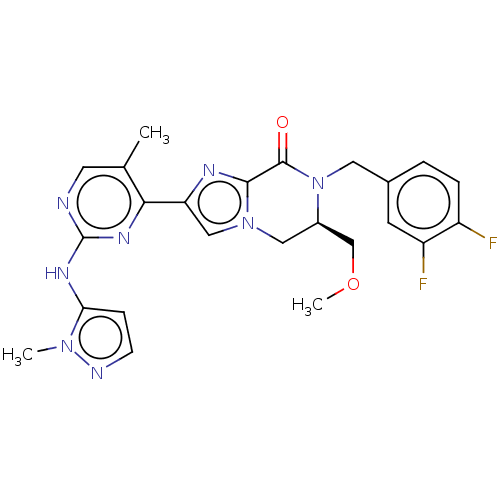
(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823
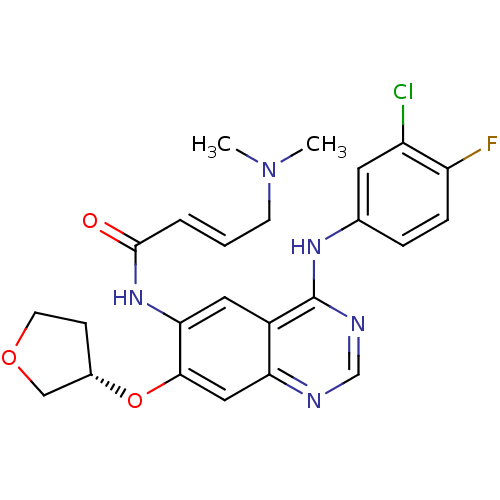
((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499
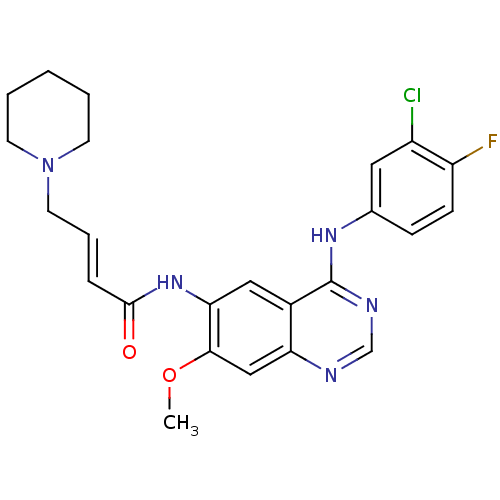
(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505990
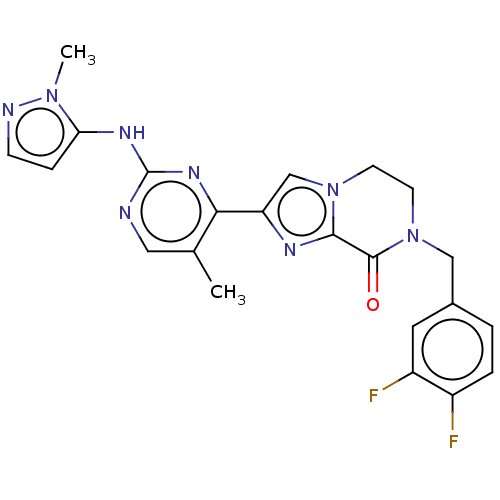
(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505990

(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029667
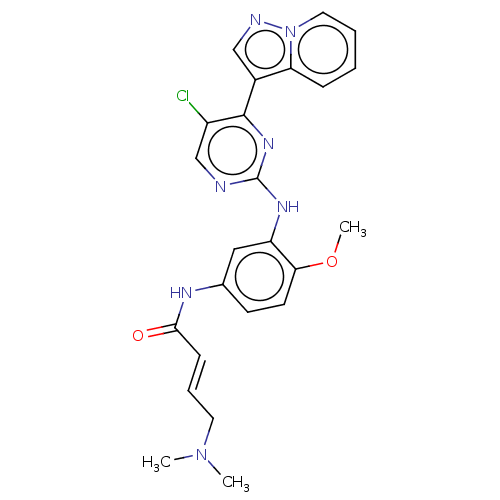
(CHEMBL2426288)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C24H24ClN7O2/c1-31(2)11-6-8-22(33)28-16-9-10-21(34-3)19(13-16)29-24-26-15-18(25)23(30-24)17-14-27-32-12-5-4-7-20(17)32/h4-10,12-15H,11H2,1-3H3,(H,28,33)(H,26,29,30)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493289
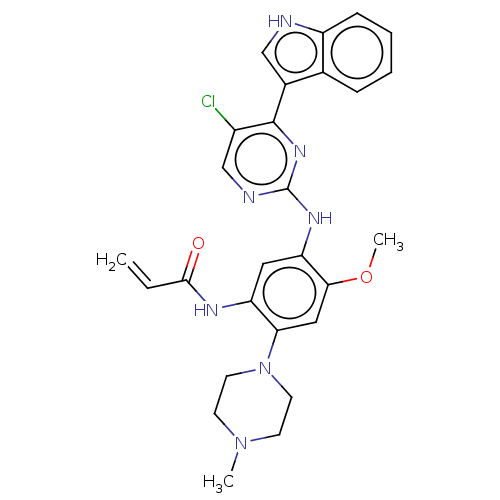
(CHEMBL2426278)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H28ClN7O2/c1-4-25(36)31-21-13-22(24(37-3)14-23(21)35-11-9-34(2)10-12-35)32-27-30-16-19(28)26(33-27)18-15-29-20-8-6-5-7-17(18)20/h4-8,13-16,29H,1,9-12H2,2-3H3,(H,31,36)(H,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of RSK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029685
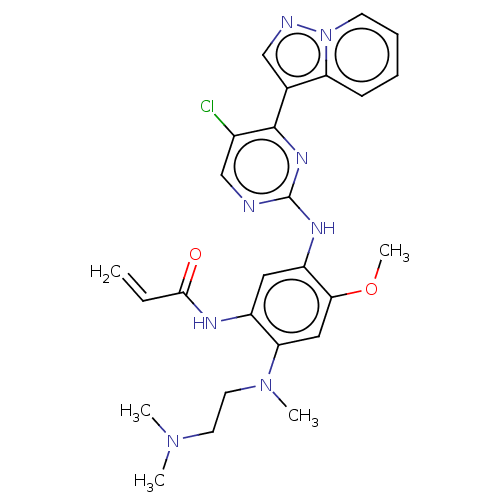
(CHEMBL3353404 | US10227342, Example 52)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H29ClN8O2/c1-6-24(36)30-19-13-20(23(37-5)14-22(19)34(4)12-11-33(2)3)31-26-28-16-18(27)25(32-26)17-15-29-35-10-8-7-9-21(17)35/h6-10,13-16H,1,11-12H2,2-5H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029684

(CHEMBL3353403)Show SMILES COc1cc(N2CC[C@H](C2)N(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 |r| Show InChI InChI=1S/C27H29ClN8O2/c1-5-25(37)31-20-12-21(24(38-4)13-23(20)35-11-9-17(16-35)34(2)3)32-27-29-15-19(28)26(33-27)18-14-30-36-10-7-6-8-22(18)36/h5-8,10,12-15,17H,1,9,11,16H2,2-4H3,(H,31,37)(H,29,32,33)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514979
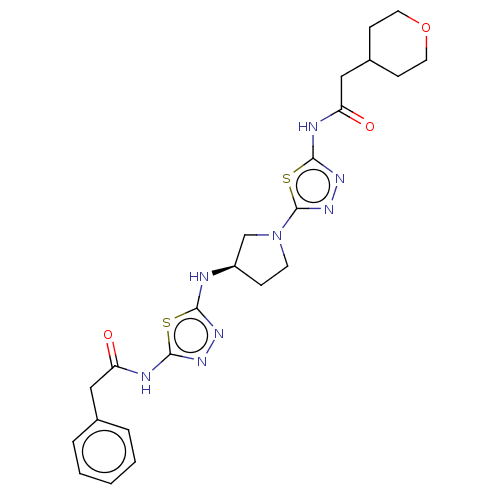
(CHEMBL4457936)Show SMILES O=C(CC1CCOCC1)Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C23H28N8O3S2/c32-18(12-15-4-2-1-3-5-15)25-21-28-27-20(35-21)24-17-6-9-31(14-17)23-30-29-22(36-23)26-19(33)13-16-7-10-34-11-8-16/h1-5,16-17H,6-14H2,(H,24,27)(H,25,28,32)(H,26,29,33)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
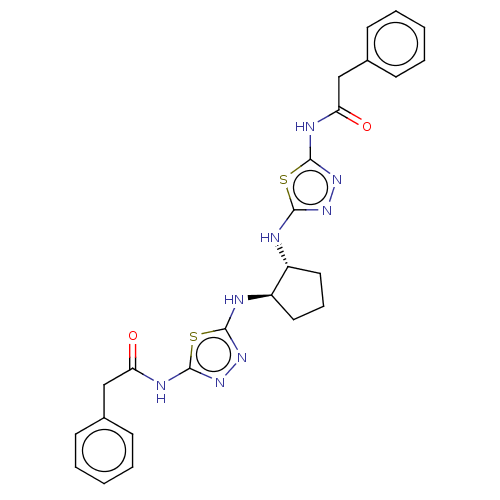
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514986
(CHEMBL4437956)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCC[C@H]2Nc2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(14-16-8-3-1-4-9-16)28-24-32-30-22(36-24)26-18-12-7-13-19(18)27-23-31-33-25(37-23)29-21(35)15-17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-15H2,(H,26,30)(H,27,31)(H,28,32,34)(H,29,33,35)/t18-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447
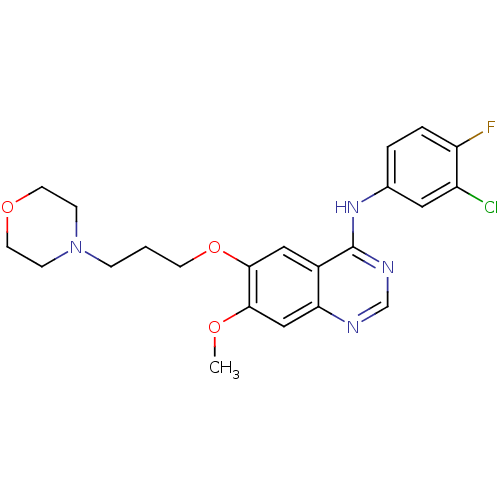
(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin receptor
(Homo sapiens (Human)) | BDBM50029685

(CHEMBL3353404 | US10227342, Example 52)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H29ClN8O2/c1-6-24(36)30-19-13-20(23(37-5)14-22(19)34(4)12-11-33(2)3)31-26-28-16-18(27)25(32-26)17-15-29-35-10-8-7-9-21(17)35/h6-10,13-16H,1,11-12H2,2-5H3,(H,30,36)(H,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of INSR (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505990

(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514985

(CHEMBL4573635)Show SMILES C[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7OS/c1-13(14-6-3-2-4-7-14)17(27)22-19-25-24-18(28-19)21-15-9-11-26(12-15)16-8-5-10-20-23-16/h2-8,10,13,15H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t13-,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of RSK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499

(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of RSK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of RSK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514975

(CHEMBL4473143)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NC[C@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(13-17-7-3-1-4-8-17)27-23-30-29-22(36-23)26-15-19-11-12-33(16-19)25-32-31-24(37-25)28-21(35)14-18-9-5-2-6-10-18/h1-10,19H,11-16H2,(H,26,29)(H,27,30,34)(H,28,31,35)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50150109
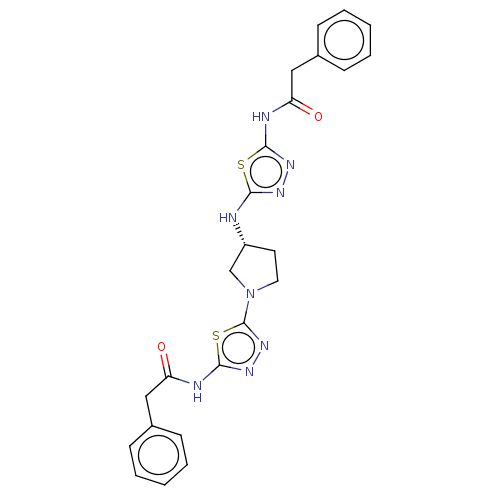
(CHEMBL3770355)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C24H24N8O2S2/c33-19(13-16-7-3-1-4-8-16)26-22-29-28-21(35-22)25-18-11-12-32(15-18)24-31-30-23(36-24)27-20(34)14-17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,25,28)(H,26,29,33)(H,27,30,34)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669
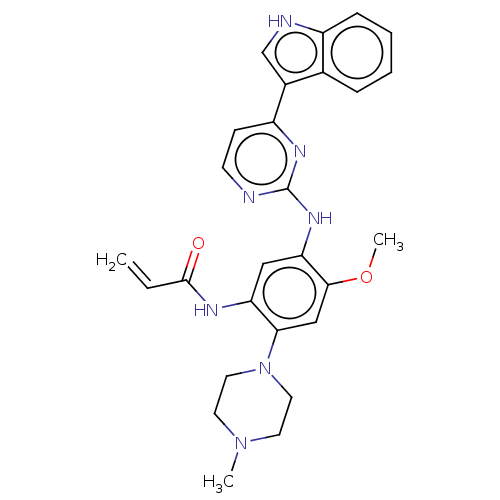
(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
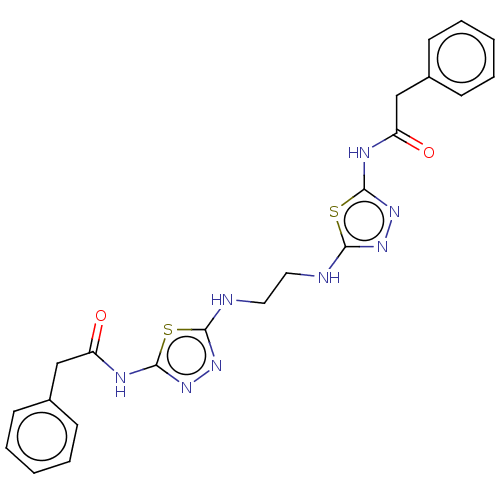
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514977
(CHEMBL4461749)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NCCNc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C22H22N8O2S2/c31-17(13-15-7-3-1-4-8-15)25-21-29-27-19(33-21)23-11-12-24-20-28-30-22(34-20)26-18(32)14-16-9-5-2-6-10-16/h1-10H,11-14H2,(H,23,27)(H,24,28)(H,25,29,31)(H,26,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669

(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514982

(CHEMBL4469711)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1 |r| Show InChI InChI=1S/C18H19N7OS/c26-16(11-13-5-2-1-3-6-13)21-18-24-23-17(27-18)20-14-8-10-25(12-14)15-7-4-9-19-22-15/h1-7,9,14H,8,10-12H2,(H,20,23)(H,21,24,26)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data