 Found 269 hits with Last Name = 'ando' and Initial = 'h'
Found 269 hits with Last Name = 'ando' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113443
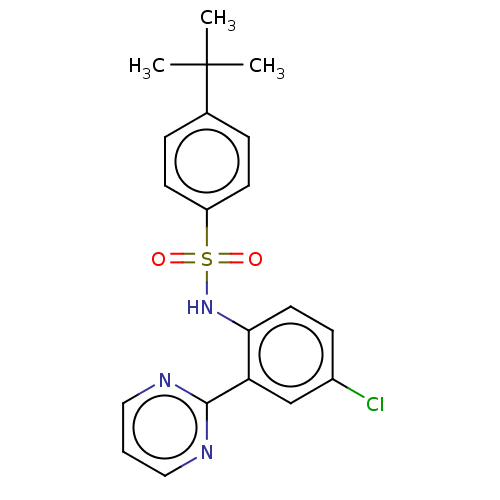
(CHEMBL3604484)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1ncccn1 Show InChI InChI=1S/C20H20ClN3O2S/c1-20(2,3)14-5-8-16(9-6-14)27(25,26)24-18-10-7-15(21)13-17(18)19-22-11-4-12-23-19/h4-13,24H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50238125
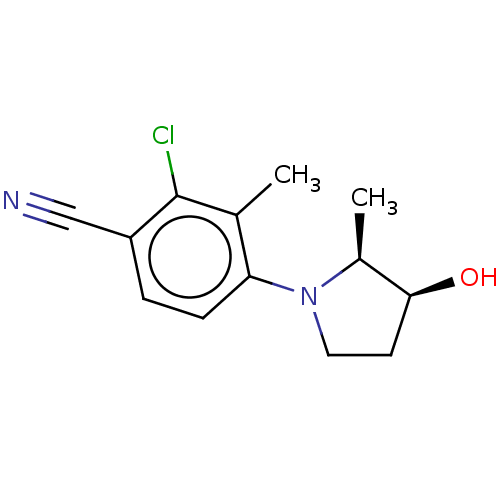
(CHEMBL4090028)Show InChI InChI=1S/C13H15ClN2O/c1-8-11(4-3-10(7-15)13(8)14)16-6-5-12(17)9(16)2/h3-4,9,12,17H,5-6H2,1-2H3/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50238127
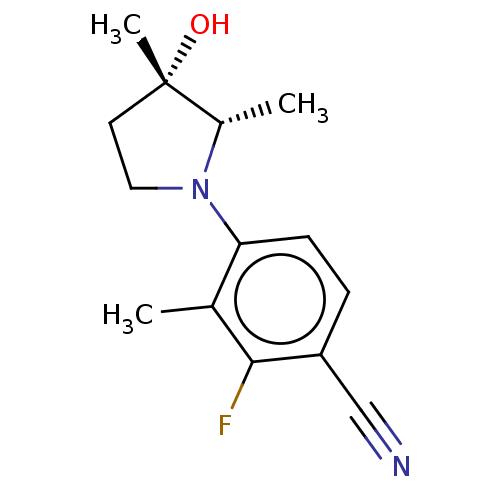
(CHEMBL4092535)Show SMILES C[C@@H]1N(CC[C@]1(C)O)c1ccc(C#N)c(F)c1C |r| Show InChI InChI=1S/C14H17FN2O/c1-9-12(5-4-11(8-16)13(9)15)17-7-6-14(3,18)10(17)2/h4-5,10,18H,6-7H2,1-3H3/t10-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Shiga/8/2004(H4N6)) neuraminidase N6 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5279173

| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242312
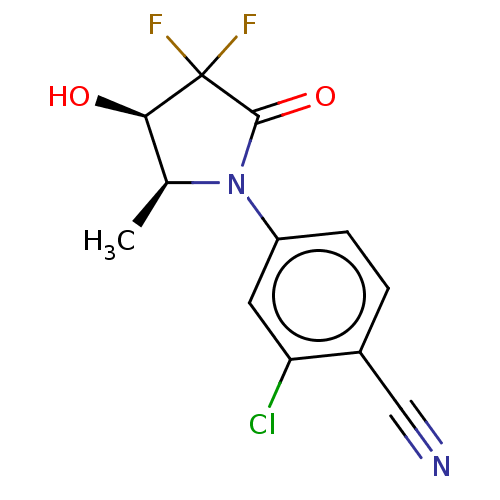
(CHEMBL4063939)Show SMILES C[C@H]1[C@@H](O)C(F)(F)C(=O)N1c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C12H9ClF2N2O2/c1-6-10(18)12(14,15)11(19)17(6)8-3-2-7(5-16)9(13)4-8/h2-4,6,10,18H,1H3/t6-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242300
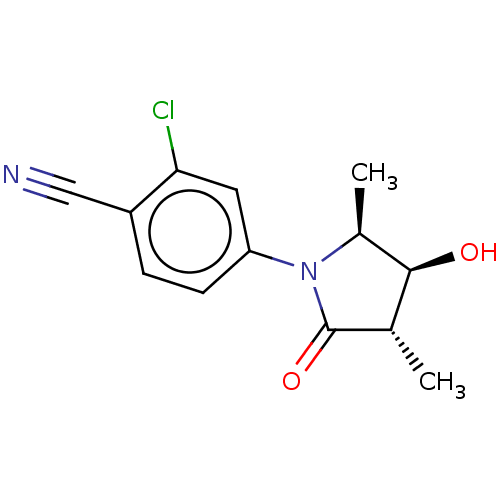
(CHEMBL4074501)Show SMILES C[C@H]1[C@@H](O)[C@H](C)C(=O)N1c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C13H13ClN2O2/c1-7-12(17)8(2)16(13(7)18)10-4-3-9(6-15)11(14)5-10/h3-5,7-8,12,17H,1-2H3/t7-,8-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50178705
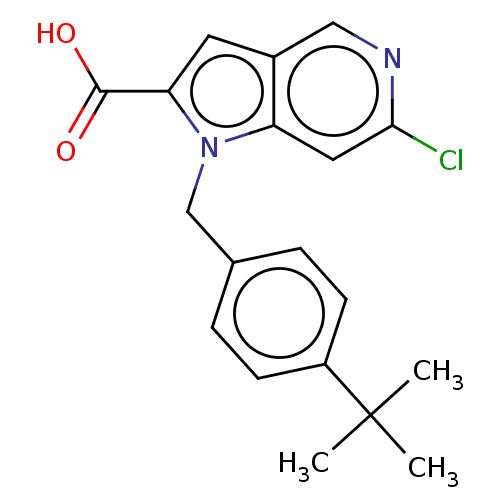
(CHEMBL3814804)Show SMILES CC(C)(C)c1ccc(Cn2c(cc3cnc(Cl)cc23)C(O)=O)cc1 Show InChI InChI=1S/C19H19ClN2O2/c1-19(2,3)14-6-4-12(5-7-14)11-22-15-9-17(20)21-10-13(15)8-16(22)18(23)24/h4-10H,11H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 (unknown origin) assessed as inhibition of TECK-stimulated calcium mobilization preincubated for 10 mins followed by agon... |
Bioorg Med Chem Lett 26: 3322-3325 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.043
BindingDB Entry DOI: 10.7270/Q2PZ5BQX |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/mallard/Hokkaido/24/2009(H5N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins f... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Narita/1/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 mins... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242303

(CHEMBL4100995)Show SMILES CC[C@H]1[C@@H](O)[C@H](C)C(=O)N1c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C14H15ClN2O2/c1-3-12-13(18)8(2)14(19)17(12)10-5-4-9(7-16)11(15)6-10/h4-6,8,12-13,18H,3H2,1-2H3/t8-,12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Aichi/102/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5288661
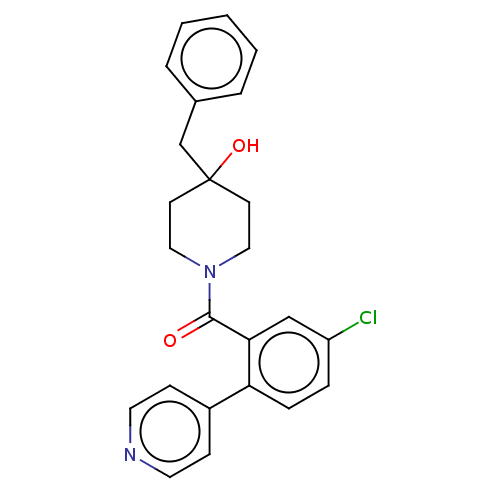
| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182248

(CHEMBL3818654)Show SMILES OC(=O)C(F)(F)F.[H][C@]1(OC(=C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)CNC(=O)C1CC1 |r,c:9| Show InChI InChI=1S/C16H25N5O7.C2HF3O2/c1-6(22)20-11-8(21-16(17)18)4-10(15(26)27)28-13(11)12(24)9(23)5-19-14(25)7-2-3-7;3-2(4,5)1(6)7/h4,7-9,11-13,23-24H,2-3,5H2,1H3,(H,19,25)(H,20,22)(H,26,27)(H4,17,18,21);(H,6,7)/t8-,9+,11+,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/Kitakyushu/10/06(H1N1) neuraminidase N1 H274Y mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 min... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5276973
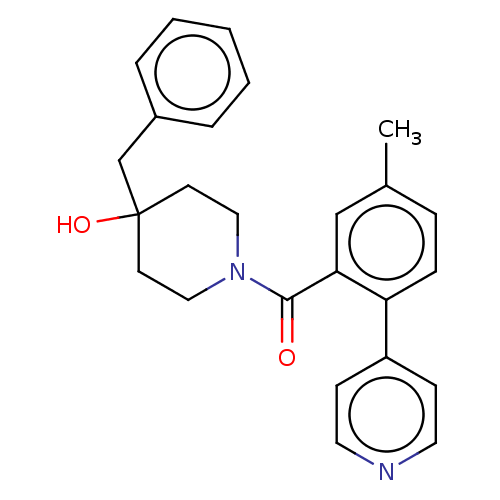
| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242301
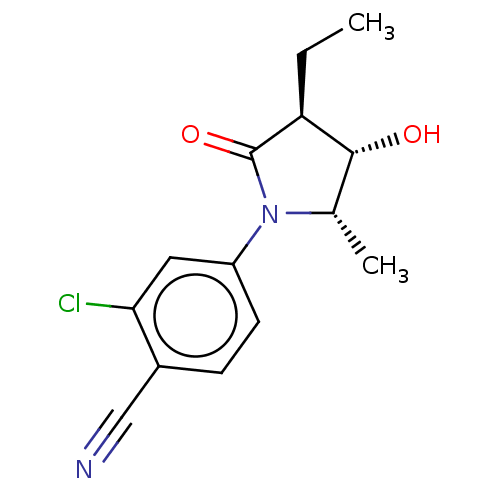
(CHEMBL4101232)Show SMILES CC[C@H]1[C@H](O)[C@H](C)N(C1=O)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C14H15ClN2O2/c1-3-11-13(18)8(2)17(14(11)19)10-5-4-9(7-16)12(15)6-10/h4-6,8,11,13,18H,3H2,1-2H3/t8-,11-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113441

(CHEMBL3604486)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1nccnc1N Show InChI InChI=1S/C20H21ClN4O2S/c1-20(2,3)13-4-7-15(8-5-13)28(26,27)25-17-9-6-14(21)12-16(17)18-19(22)24-11-10-23-18/h4-12,25H,1-3H3,(H2,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113442
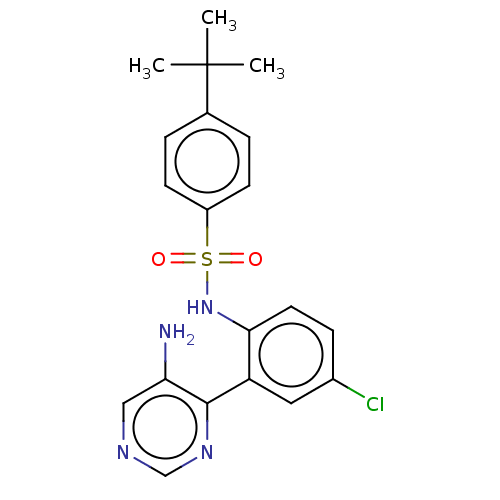
(CHEMBL3604485)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1ncncc1N Show InChI InChI=1S/C20H21ClN4O2S/c1-20(2,3)13-4-7-15(8-5-13)28(26,27)25-18-9-6-14(21)10-16(18)19-17(22)11-23-12-24-19/h4-12,25H,22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242309
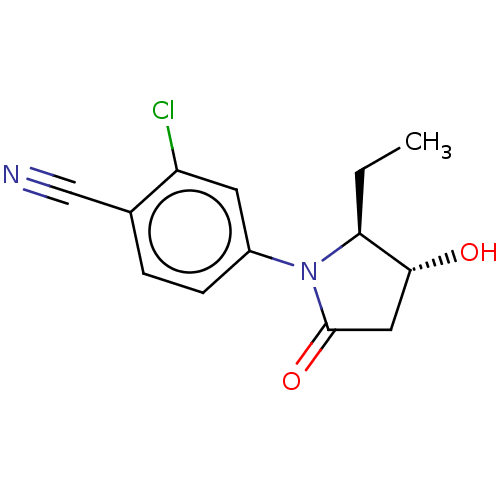
(CHEMBL4067691)Show SMILES CC[C@H]1[C@H](O)CC(=O)N1c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C13H13ClN2O2/c1-2-11-12(17)6-13(18)16(11)9-4-3-8(7-15)10(14)5-9/h3-5,11-12,17H,2,6H2,1H3/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242297
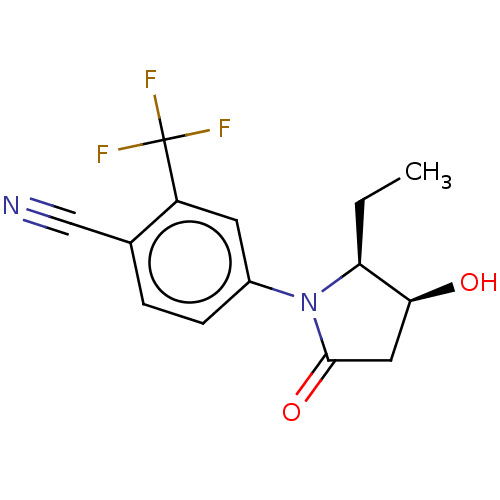
(CHEMBL4088914)Show SMILES CC[C@H]1[C@@H](O)CC(=O)N1c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N2O2/c1-2-11-12(20)6-13(21)19(11)9-4-3-8(7-18)10(5-9)14(15,16)17/h3-5,11-12,20H,2,6H2,1H3/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113352
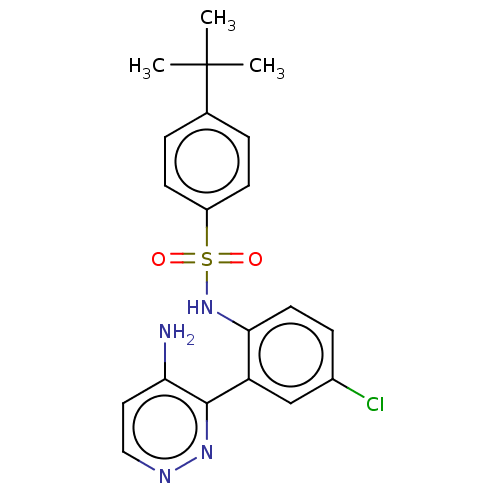
(CHEMBL3604487)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1nnccc1N Show InChI InChI=1S/C20H21ClN4O2S/c1-20(2,3)13-4-7-15(8-5-13)28(26,27)25-18-9-6-14(21)12-16(18)19-17(22)10-11-23-24-19/h4-12,25H,1-3H3,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50242308
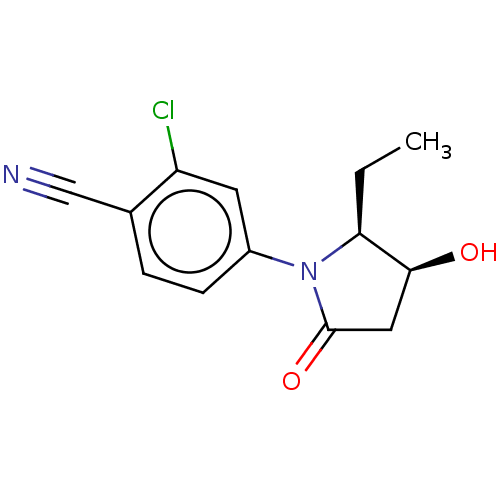
(CHEMBL4072533)Show SMILES CC[C@H]1[C@@H](O)CC(=O)N1c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C13H13ClN2O2/c1-2-11-12(17)6-13(18)16(11)9-4-3-8(7-15)10(14)5-9/h3-5,11-12,17H,2,6H2,1H3/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from human AR after 3 hrs |
Bioorg Med Chem 25: 3330-3349 (2017)
Article DOI: 10.1016/j.bmc.2017.04.018
BindingDB Entry DOI: 10.7270/Q2PV6NSW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50113354
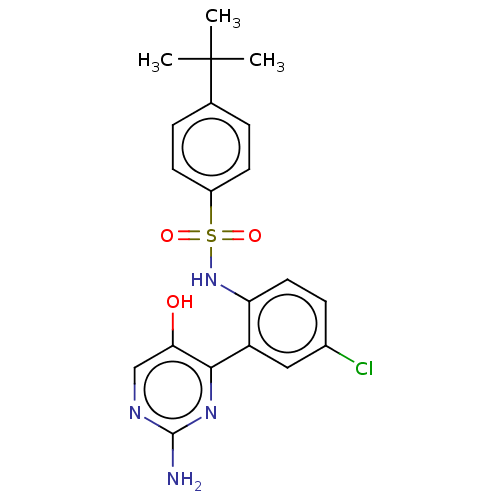
(CHEMBL3604489)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1-c1nc(N)ncc1O Show InChI InChI=1S/C20H21ClN4O3S/c1-20(2,3)12-4-7-14(8-5-12)29(27,28)25-16-9-6-13(21)10-15(16)18-17(26)11-23-19(22)24-18/h4-11,25-26H,1-3H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 receptor (unknown origin) assessed as inhibition of TECK-induced calcium mobilization incubated for 10 mins prior to TECK... |
Bioorg Med Chem Lett 25: 3661-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.046
BindingDB Entry DOI: 10.7270/Q2RR2123 |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5282804
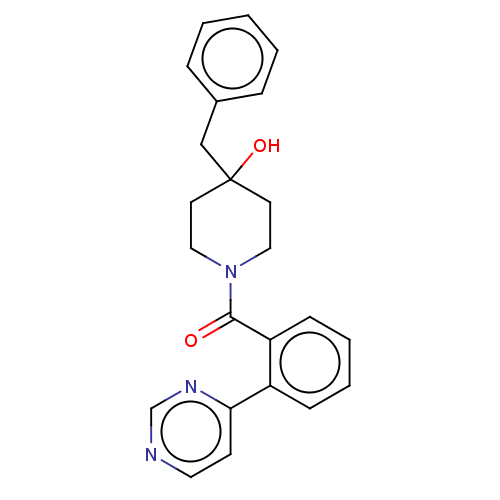
| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
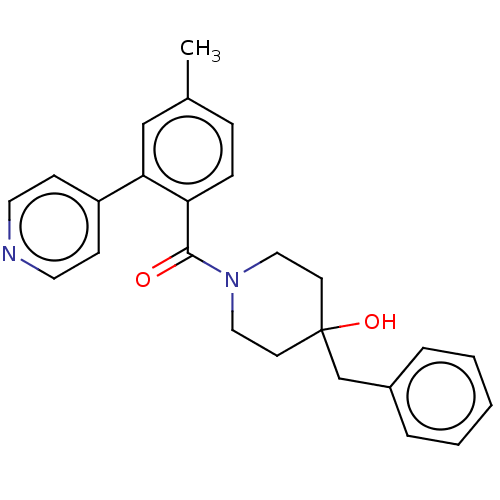
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5271033
| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182248

(CHEMBL3818654)Show SMILES OC(=O)C(F)(F)F.[H][C@]1(OC(=C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)CNC(=O)C1CC1 |r,c:9| Show InChI InChI=1S/C16H25N5O7.C2HF3O2/c1-6(22)20-11-8(21-16(17)18)4-10(15(26)27)28-13(11)12(24)9(23)5-19-14(25)7-2-3-7;3-2(4,5)1(6)7/h4,7-9,11-13,23-24H,2-3,5H2,1H3,(H,19,25)(H,20,22)(H,26,27)(H4,17,18,21);(H,6,7)/t8-,9+,11+,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
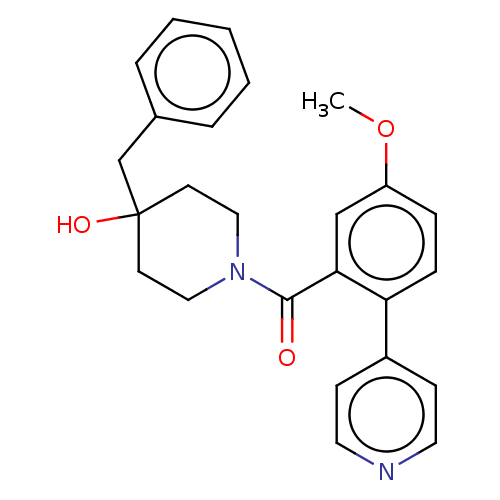
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5269674
| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | CHEMBL5289771

| PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50182249

(CHEMBL3818159)Show SMILES OP(O)(O)=O.CCC(CC)OC[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N2O4.H3O4P/c1-4-12(5-2)21-8-11-6-10(15(19)20)7-13(16)14(11)17-9(3)18;1-5(2,3)4/h7,11-14H,4-6,8,16H2,1-3H3,(H,17,18)(H,19,20);(H3,1,2,3,4)/t11-,13-,14-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | |
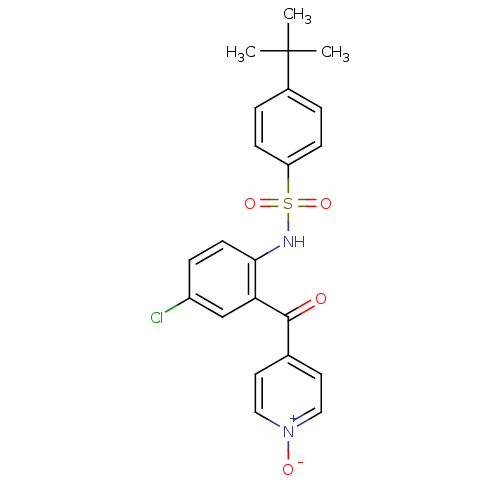
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50398334
(CHEMBL2178578)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1C(=O)c1cc[n+]([O-])cc1 Show InChI InChI=1S/C22H21ClN2O4S/c1-22(2,3)16-4-7-18(8-5-16)30(28,29)24-20-9-6-17(23)14-19(20)21(26)15-10-12-25(27)13-11-15/h4-14,24H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 (unknown origin) assessed as inhibition of TECK-stimulated calcium mobilization preincubated for 10 mins followed by agon... |
Bioorg Med Chem Lett 26: 3322-3325 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.043
BindingDB Entry DOI: 10.7270/Q2PZ5BQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Tsukuba/28/2006(H3N8)) neuraminidase N8 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... |
J Med Chem 59: 4563-77 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01863
BindingDB Entry DOI: 10.7270/Q2QN68QP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data