

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50007002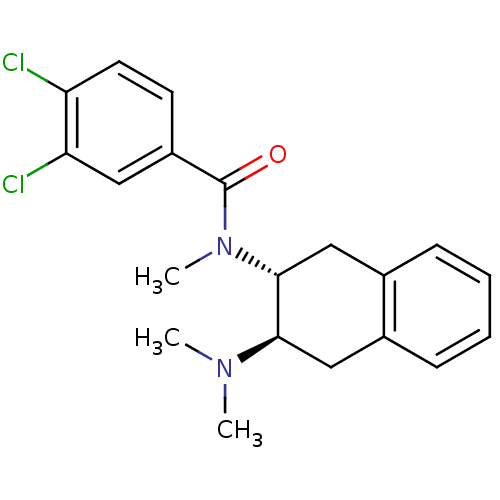 (3,4-Dichloro-N-(3-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50229403 (CHEMBL2311130) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Compound was evaluated for time-dependent inactivation of Ribonucleotide diphosphate reductase (RDPR) in E. coli | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006996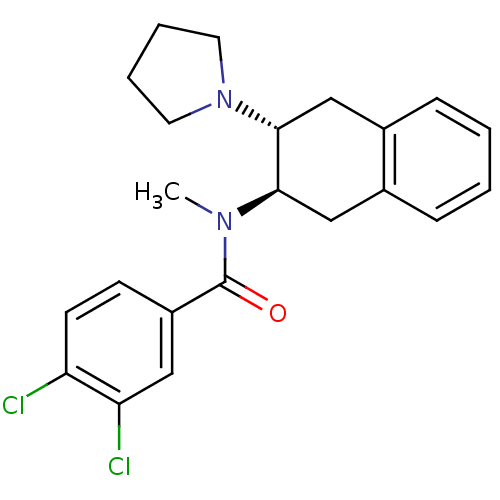 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007004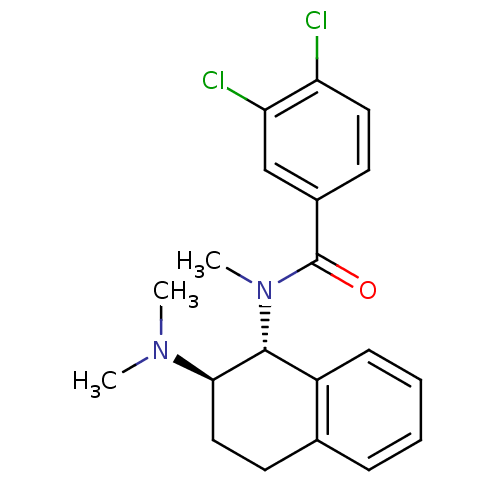 (3,4-Dichloro-N-(2-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006997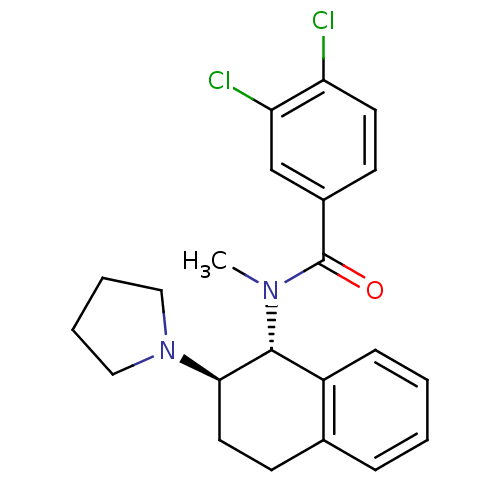 (3,4-Dichloro-N-methyl-N-(2-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006994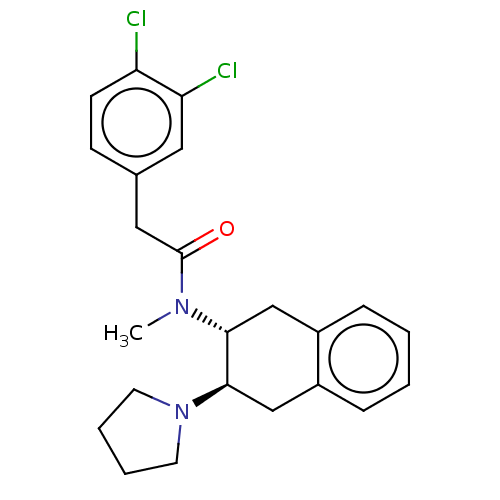 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006999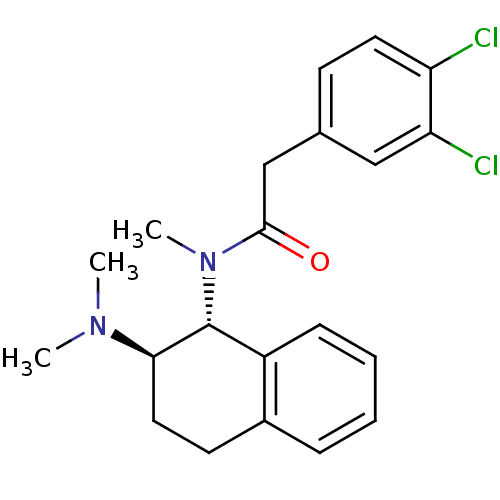 (2-(3,4-Dichloro-phenyl)-N-(2-dimethylamino-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007001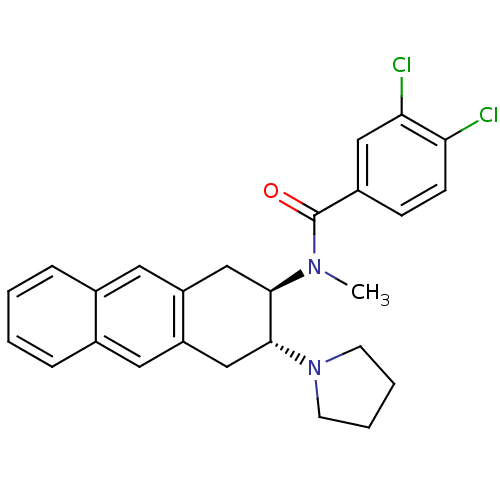 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006998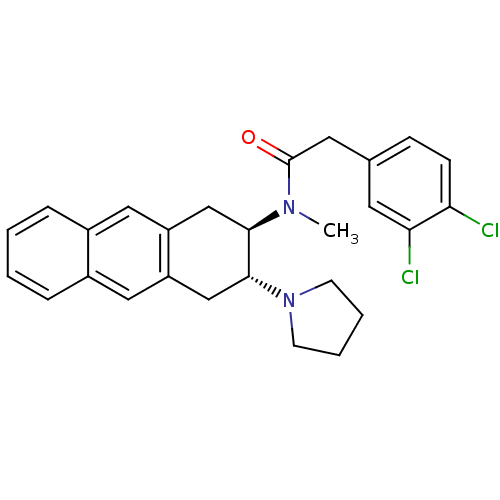 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007000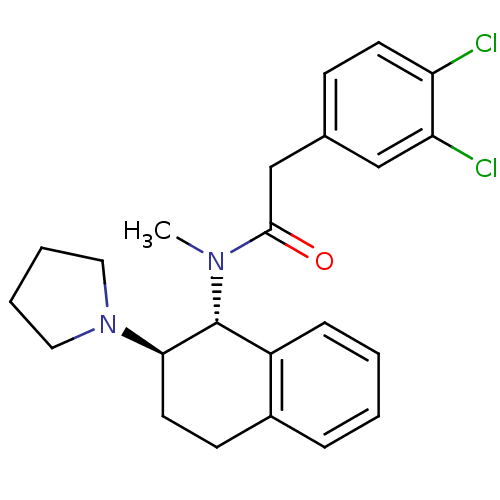 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534094 (WO2022081807, Example 201) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287622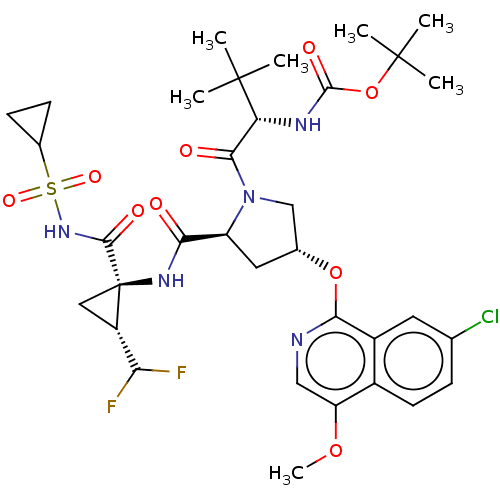 (CHEMBL4160876) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287594 (Asunaprevir | BMS 650032 | BMS-650032) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM533971 (WO2022081807, Example 78) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM533872 (WO2022081807, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534119 (WO2022081807, Example 226) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534154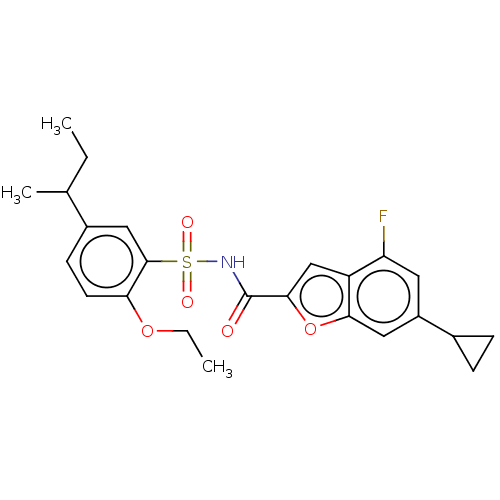 (6-Cyclopropyl-N-(2-ethoxy-5-sec-butyl-phenyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534100 (WO2022081807, Example 207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534192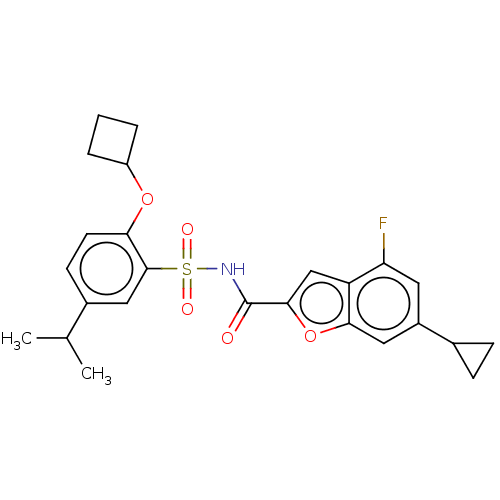 (N-[(2-Cyclobutoxy-5-isopropylphenyl)sulfonyl]-6-cy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534190 (N-((5-(tert-Butyl)-2-cyclobutoxyphenyl)sulfonyl)-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534121 (WO2022081807, Example 228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534187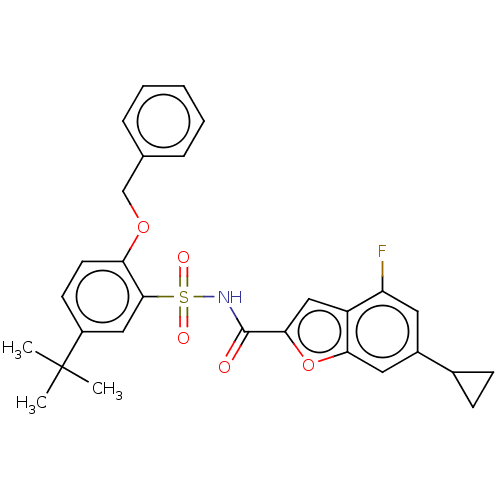 (N-[(2-(Benzyloxy)-5-(tert-butyl)phenyl)sulfonyl]-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 2.67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534188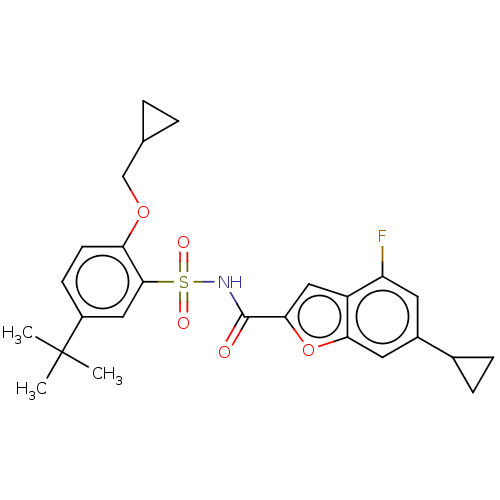 (N-((5-(tert-butyl)-2-(cyclopropylmethoxy)phenyl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 2.92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534161 (6-Cyclopropyl-N-[2-(cyclopropylmethoxy)-5-isopropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 3.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534193 (N-((5-(tert-butyl)-2-methoxyphenyl)sulfonyl)-6-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534186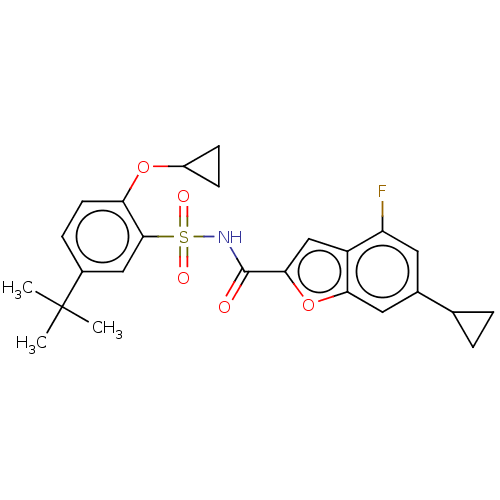 (N-((5-(tert-butyl)-2-cyclopropoxyphenyl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 3.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534117 (WO2022081807, Example 224) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534118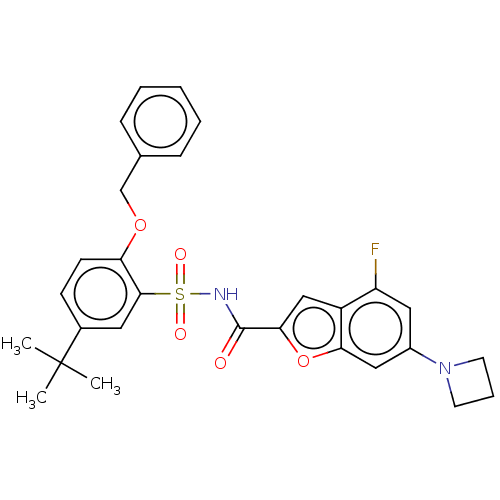 (6-(Azetidin-1-yl)-N-[(2-(benzyloxy)-5-(tert-butyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534107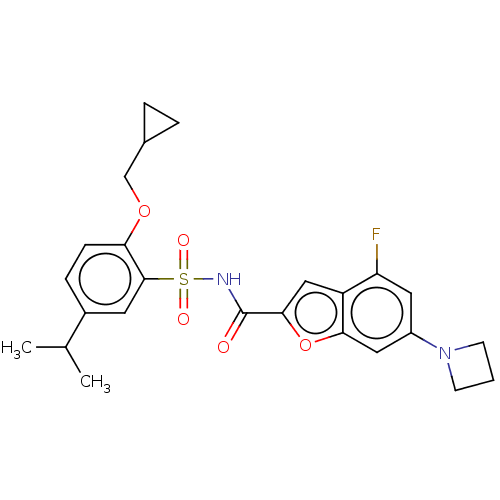 (6-(azetidin-1-yl)-N-[2-(cyclopropylmethoxy)-5-isop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 3.91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM533874 (WO2022081807, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534189 (N-((5-(tert-butyl)-2-(2,2,2-trifluoroethoxy)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534087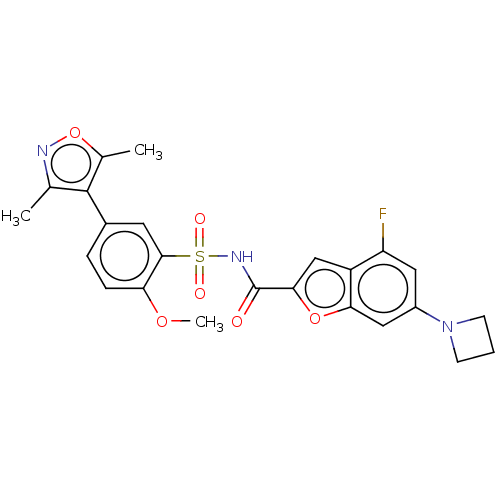 (6-(Azetidin-1-yl)-N-[5-(3,5-dimethyl-1,2-oxazol-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534124 (6-(Azetidin-1-yl)-N-((2-((2,2-difluorocyclopropyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534043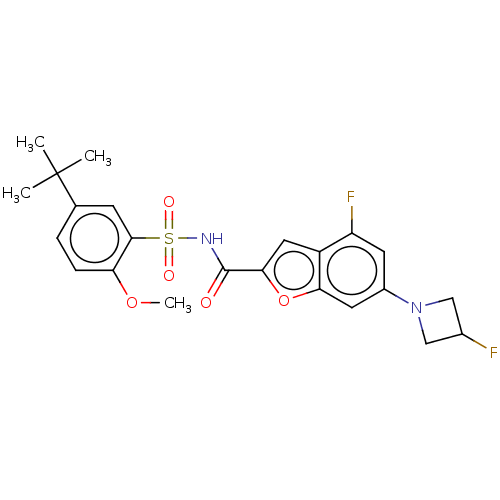 (WO2022081807, Example 150) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534191 (N-((5-(tert-Butyl)-2-isopropoxyphenyl)sulfonyl)-6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM533884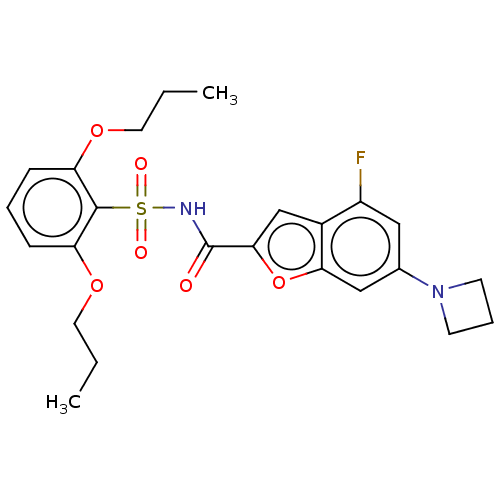 (6-(Azetidin-1-yl)-N-(2,6-dipropoxybenzene-1-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534006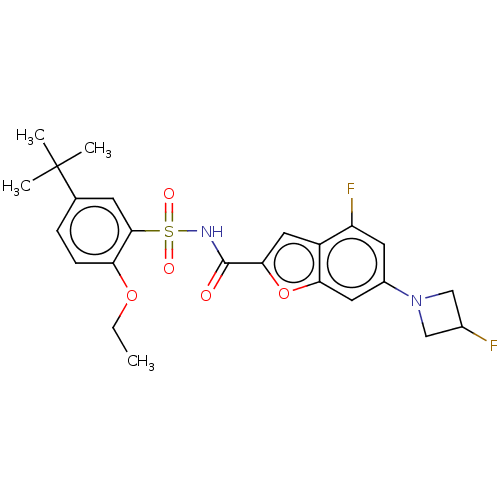 (N-(5-tert-butyl-2-ethoxybenzene-1-sulfonyl)-4-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534194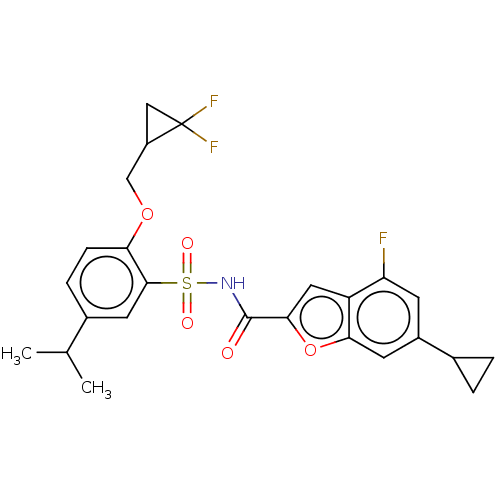 (6-Cyclopropyl-N-{(2-[(2,2-difluorocyclopropyl)meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534020 (6-(Azetidin-1-yl)-N-(5-tert-butyl-2-methoxybenzene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534174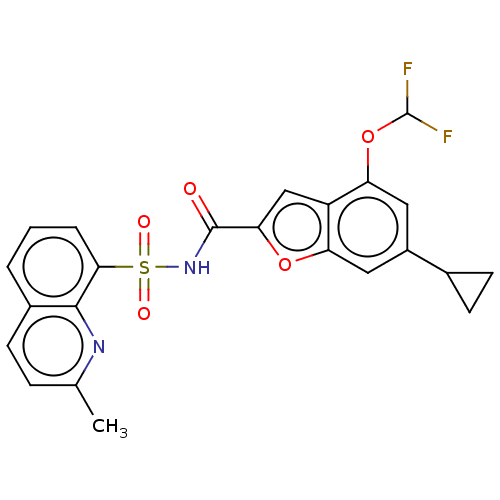 (6-Cyclopropyl-4-(difluoromethoxy)-N-[(2-methyl-8-q...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534122 (6-(Azetidin-1-yl)-N-[(5-(tert-butyl)-2-isopropoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534195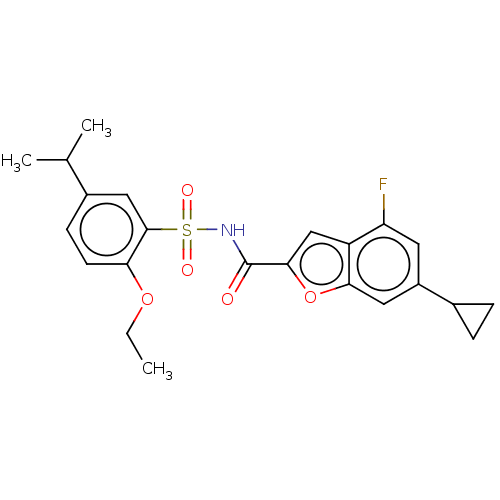 (6-Cyclopropyl-N-(2-ethoxy-5-isopropyl-phenyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534168 (6-Cyclopropyl-4-fluoro-N-[5-isopropyl-2-(2,2,2-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 4.99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287595 (CHEMBL3921126) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534088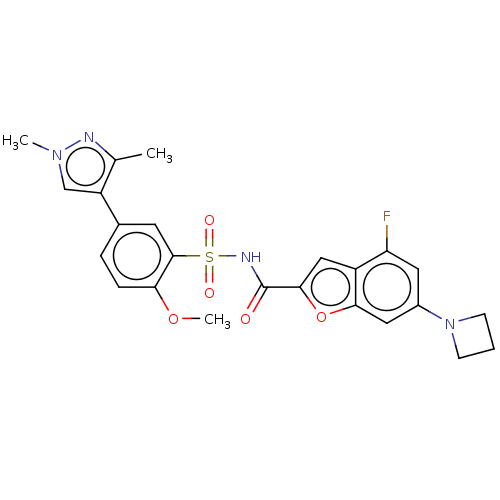 (WO2022081807, Example 195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 5.07 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006994 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534120 (6-(Azetidin-1-yl)-N-[(5-(tert-butyl)-2-(2,2,2-trif...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081807 | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM534177 (6-Cyclopropyl-N-[(2-methyl-8-quinolyl)sulfonyl]-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022081842 | n/a | n/a | 5.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4GFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 461 total ) | Next | Last >> |