

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Mus musculus) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against L929 cells AdoHcy hydrolase activity | J Med Chem 37: 551-4 (1994) BindingDB Entry DOI: 10.7270/Q2DJ5DQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50034176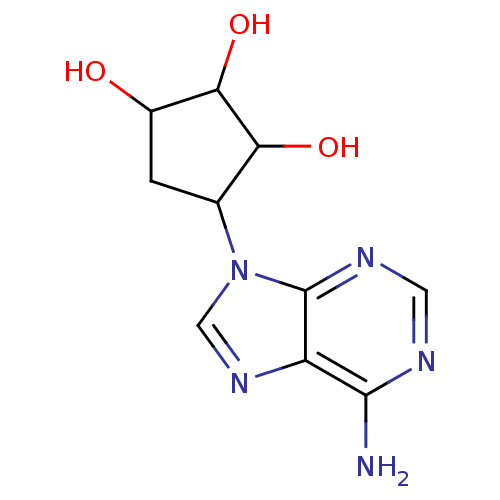 (4-(6-Amino-purin-9-yl)-cyclopentane-1,2,3-triol | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against L929 cells AdoHcy hydrolase activity | J Med Chem 37: 551-4 (1994) BindingDB Entry DOI: 10.7270/Q2DJ5DQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50008288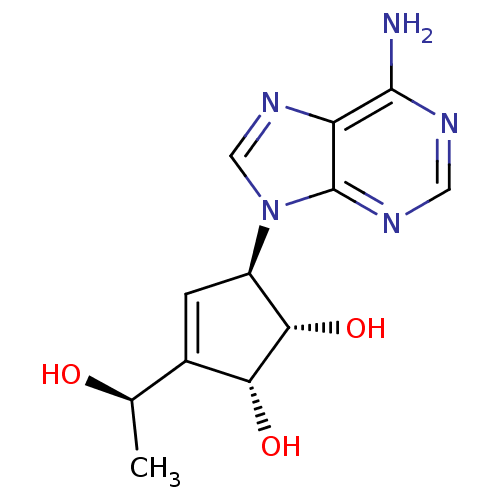 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((R)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50006223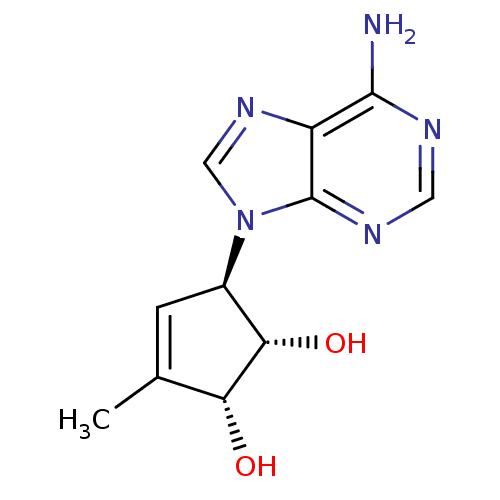 (5-(6-Amino-purin-9-yl)-3-methyl-cyclopent-3-ene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490649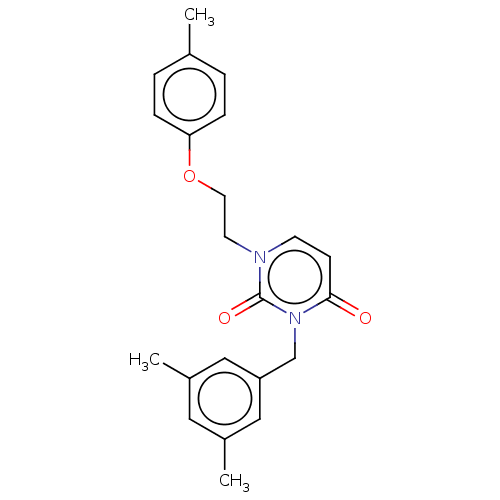 (CHEMBL2337184) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490644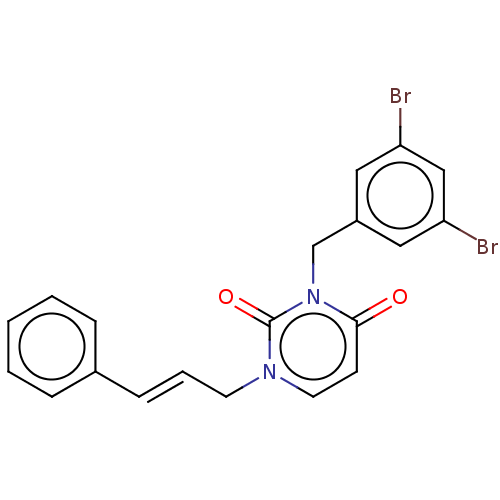 (CHEMBL2337195) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490643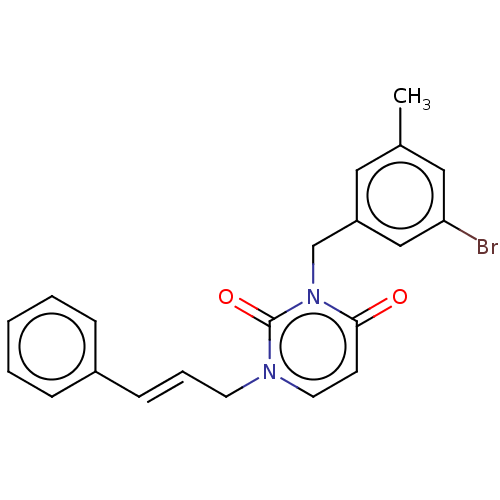 (CHEMBL2337196) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490669 (CHEMBL2337192) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490662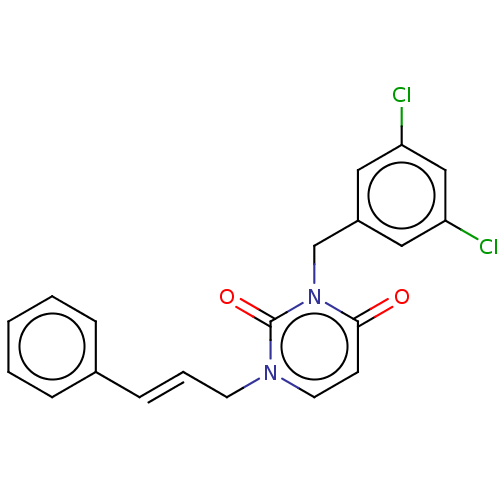 (CHEMBL2337194) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490668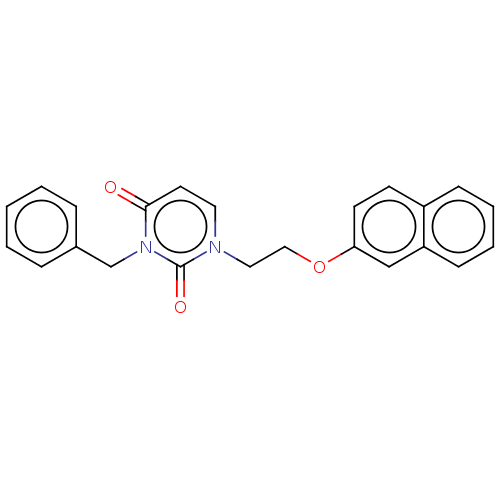 (CHEMBL2337189) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490648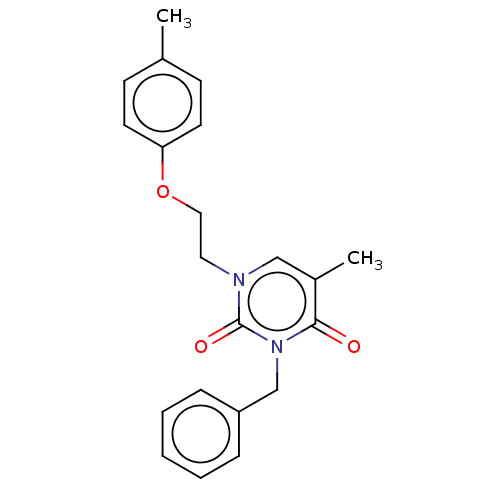 (CHEMBL2337186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490646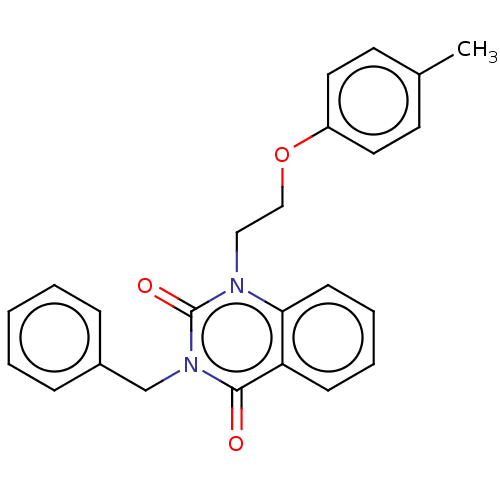 (CHEMBL2337190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369380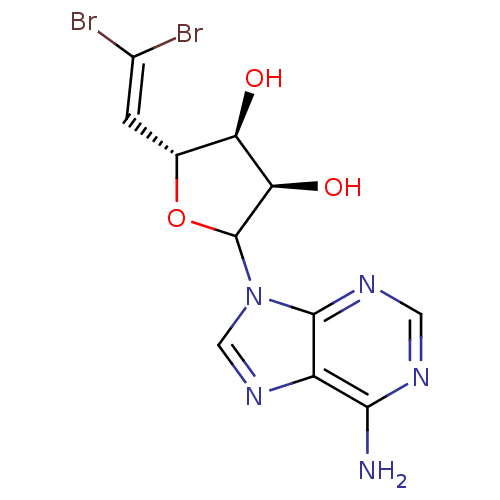 (CHEMBL606502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound | J Med Chem 41: 3078-83 (1998) Article DOI: 10.1021/jm9801410 BindingDB Entry DOI: 10.7270/Q2MC90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369381 (CHEMBL612194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound | J Med Chem 41: 3078-83 (1998) Article DOI: 10.1021/jm9801410 BindingDB Entry DOI: 10.7270/Q2MC90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369379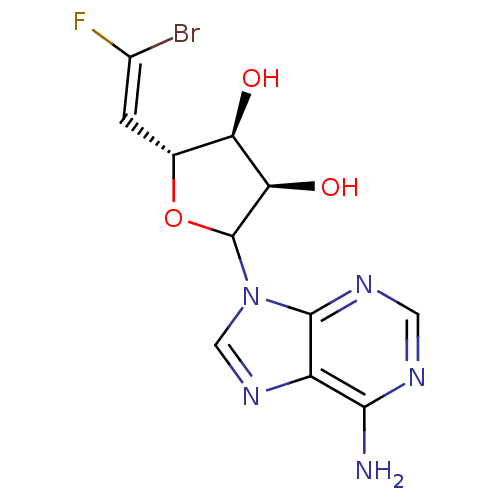 (CHEMBL607755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound | J Med Chem 41: 3078-83 (1998) Article DOI: 10.1021/jm9801410 BindingDB Entry DOI: 10.7270/Q2MC90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490660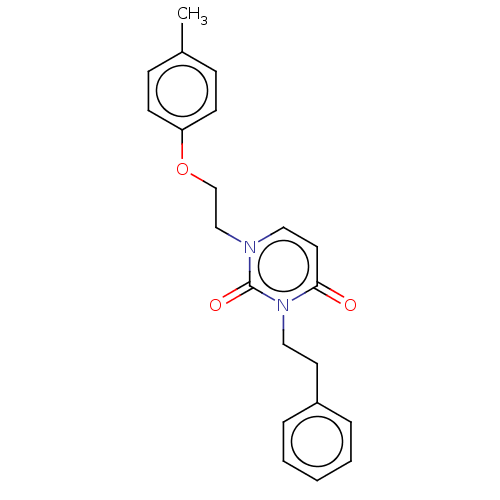 (CHEMBL2337187) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490665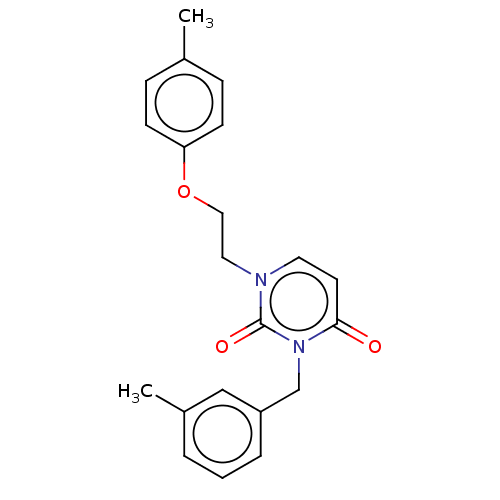 (CHEMBL2337181) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490642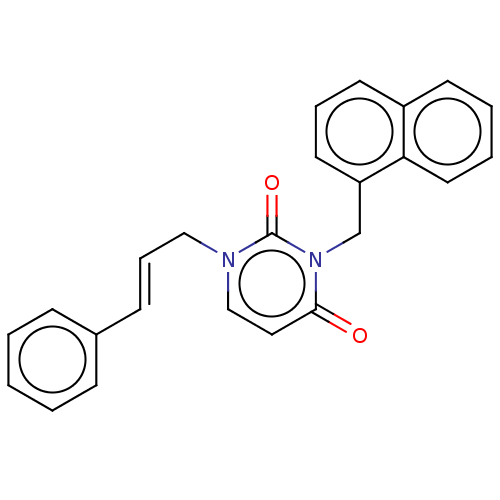 (CHEMBL2337197) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490659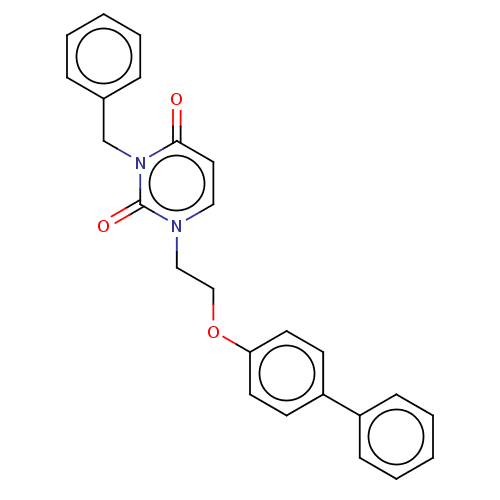 (CHEMBL2337174) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490650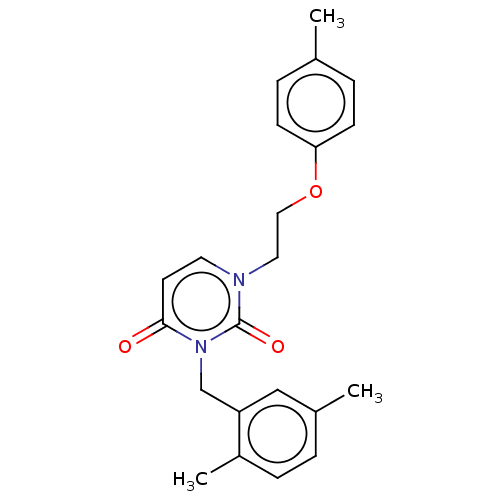 (CHEMBL2337183) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490647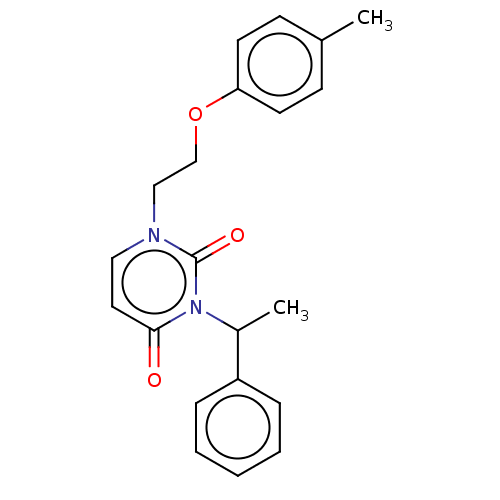 (CHEMBL2337188) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490645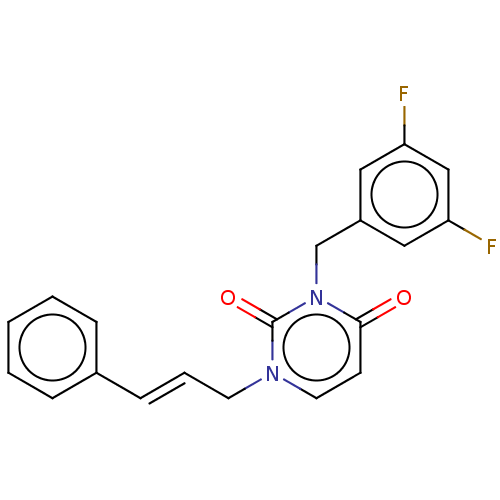 (CHEMBL2337193) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490653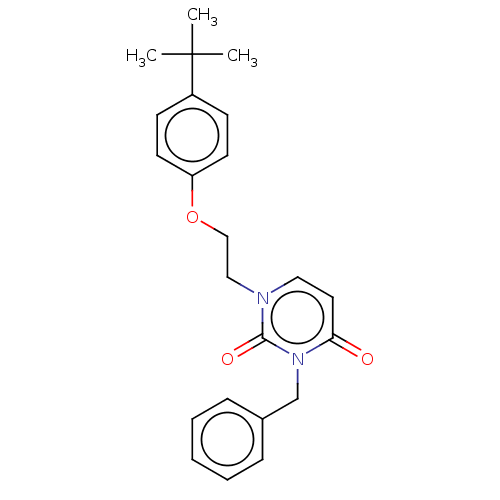 (CHEMBL2337173) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490658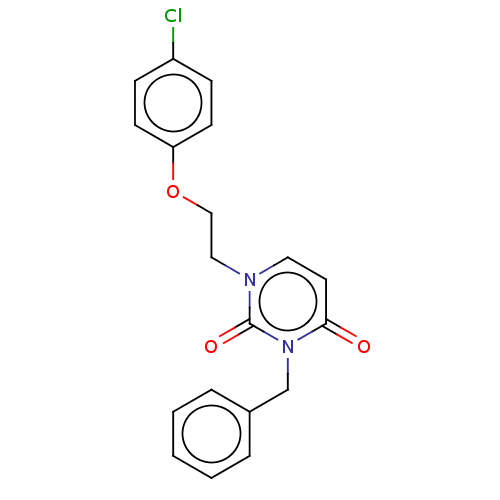 (CHEMBL2337175) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490663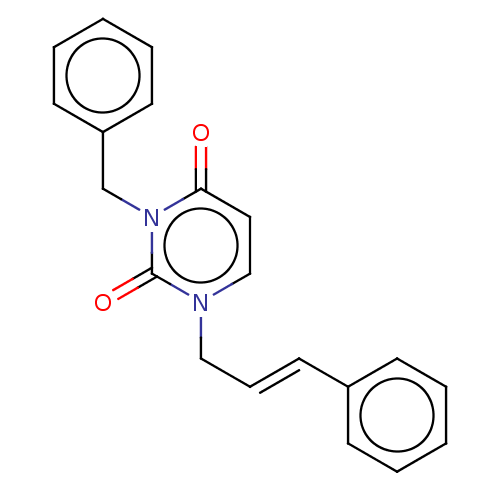 (CHEMBL2337191) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490657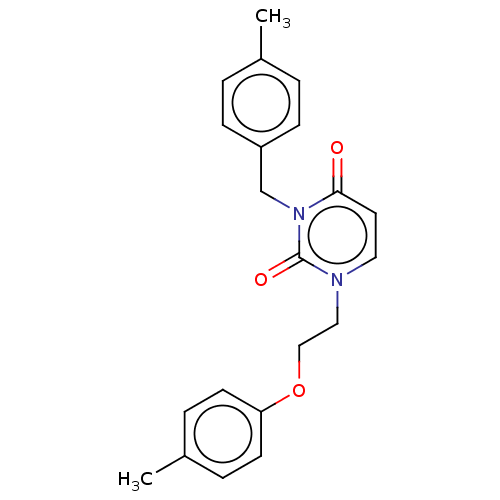 (CHEMBL2337182) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490652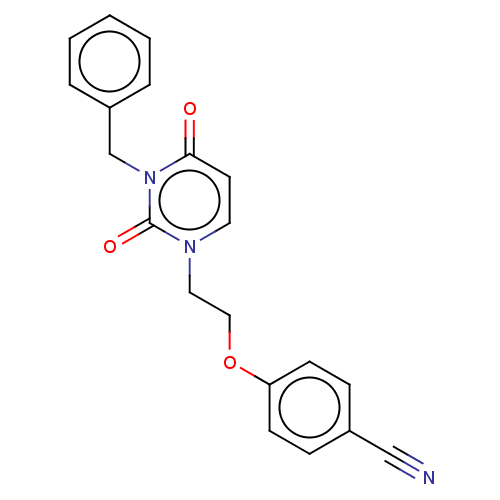 (CHEMBL2337177) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490654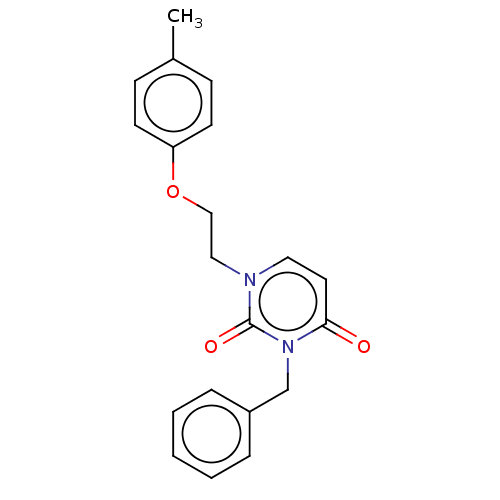 (CHEMBL2337201) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50008289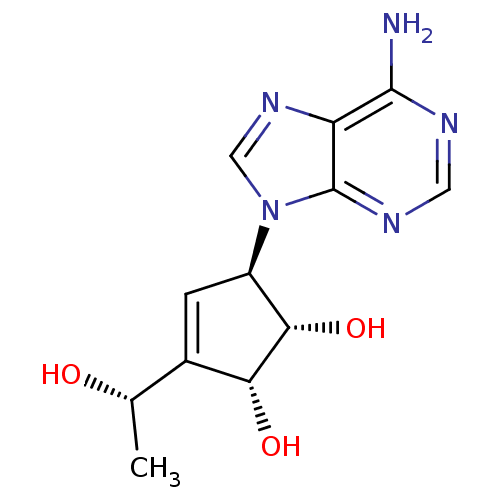 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((S)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against L929 Cell S-adenosyl-L-homocysteine hydrolase | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490656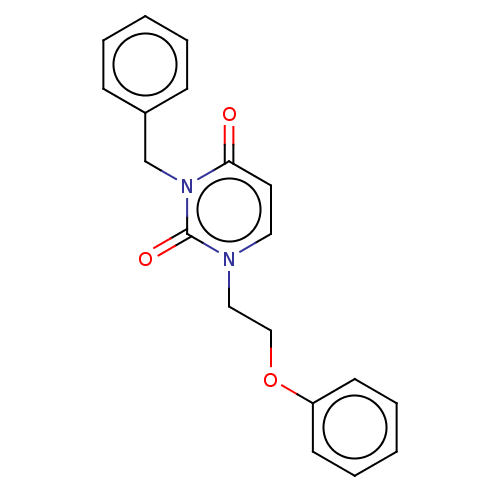 (CHEMBL2337198) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490661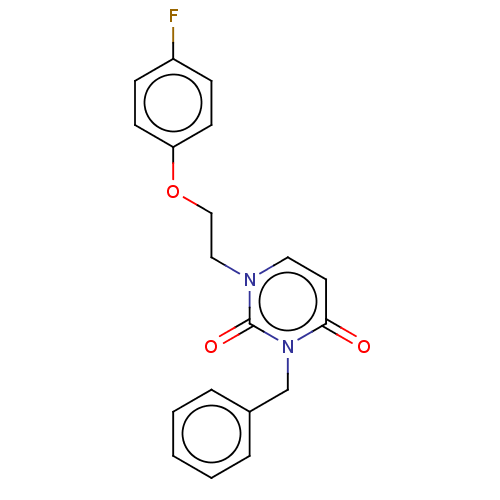 (CHEMBL2337176) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490664 (CHEMBL2337185) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490666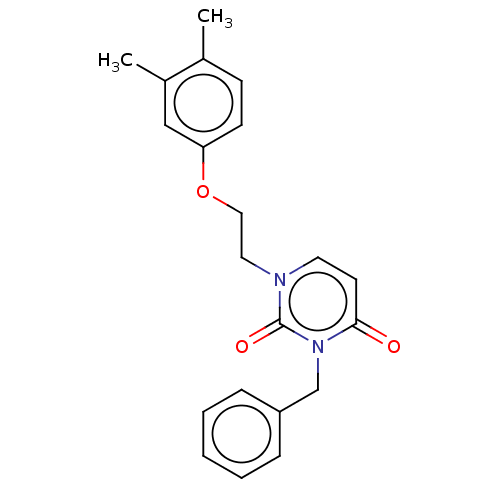 (CHEMBL2337178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490667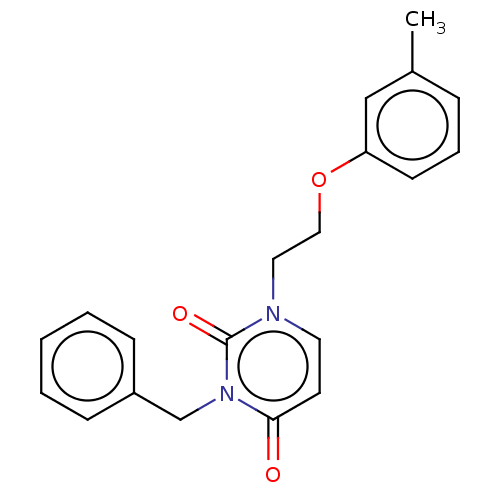 (CHEMBL2337200) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490651 (CHEMBL2337179) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490670 (CHEMBL2337180) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490655 (CHEMBL2337199) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase | Bioorg Med Chem Lett 3: 663-666 (1993) Article DOI: 10.1016/S0960-894X(01)81249-X BindingDB Entry DOI: 10.7270/Q2X92BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory activity against S-adenosyl-L-homocysteine hydrolase from rabbit erythrocytes | J Med Chem 39: 2392-9 (1996) Article DOI: 10.1021/jm950853f BindingDB Entry DOI: 10.7270/Q2GM8B1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50281613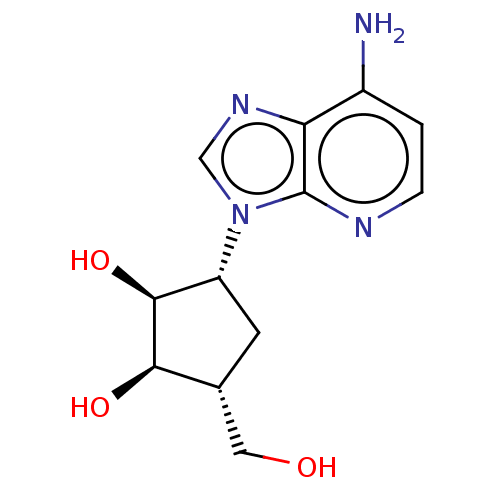 (3-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-5-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase | Bioorg Med Chem Lett 3: 663-666 (1993) Article DOI: 10.1016/S0960-894X(01)81249-X BindingDB Entry DOI: 10.7270/Q2X92BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50281612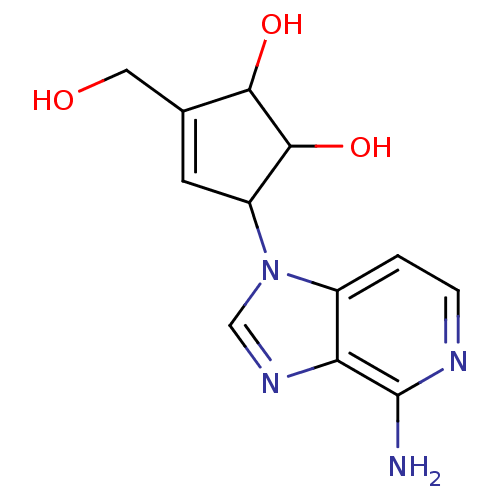 (5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-3-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase | Bioorg Med Chem Lett 3: 663-666 (1993) Article DOI: 10.1016/S0960-894X(01)81249-X BindingDB Entry DOI: 10.7270/Q2X92BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50366323 ((S)-DHPA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory concentration against S-adenosyl-homocysteine hydrolase | Bioorg Med Chem Lett 3: 663-666 (1993) Article DOI: 10.1016/S0960-894X(01)81249-X BindingDB Entry DOI: 10.7270/Q2X92BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50008288 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-((R)-1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyo Jozo Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect against S-adenosyl-L-homocysteine hydrolase of rabbit erythrocyte. | J Med Chem 35: 324-31 (1992) BindingDB Entry DOI: 10.7270/Q2RX9B1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50335554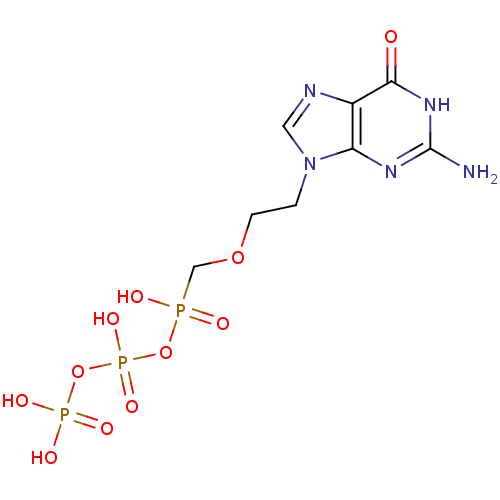 (({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA. Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta by microplate reader analysis | Antimicrob Agents Chemother 53: 2777-84 (2009) Article DOI: 10.1128/AAC.00103-09 BindingDB Entry DOI: 10.7270/Q2M32W2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | CHEMBL5287352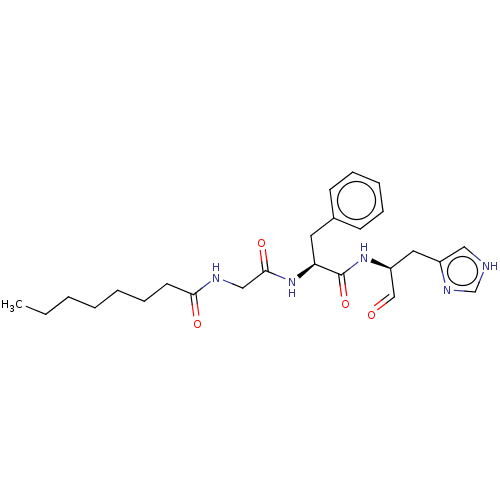 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50084655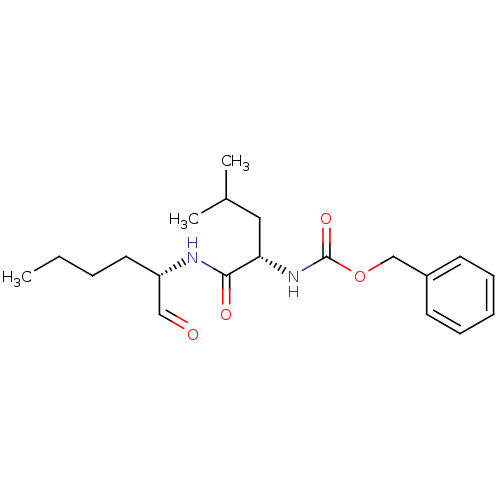 (CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50335554 (({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA. Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha by microplate reader analysis | Antimicrob Agents Chemother 53: 2777-84 (2009) Article DOI: 10.1128/AAC.00103-09 BindingDB Entry DOI: 10.7270/Q2M32W2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 412 total ) | Next | Last >> |