

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136366 ((S)-Piperidine-3-carboxylic acid [(R)-1-(4-chloro-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136365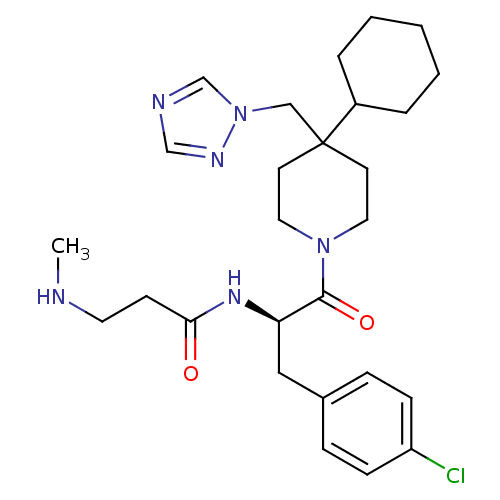 (CHEMBL337571 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136357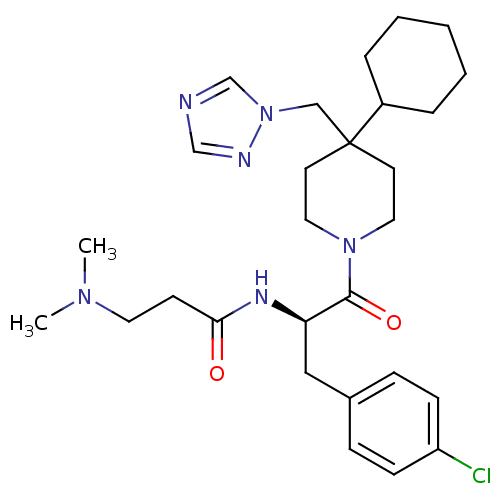 (CHEMBL341982 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136372 (CHEMBL343076 | N-{2-[(R)-1-(4-Chloro-benzyl)-2-(4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136367 (CHEMBL137273 | N-{2-[(R)-1-(4-Chloro-benzyl)-2-(4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136360 (3-Acetylamino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cycl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136364 ((R)-Piperidine-3-carboxylic acid [(R)-1-(4-chloro-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136370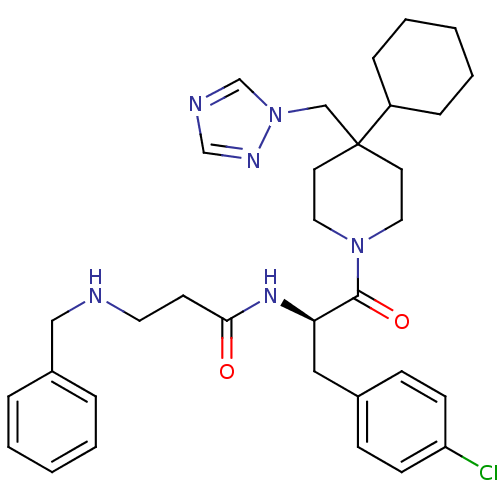 (3-Benzylamino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cycl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136355 (3-Amino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Stimulation of adenylate cyclase in HEK293 cells expressing the human MC4R receptor was determined by measuring cAMP accumulation using the RPA559 SP... | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136368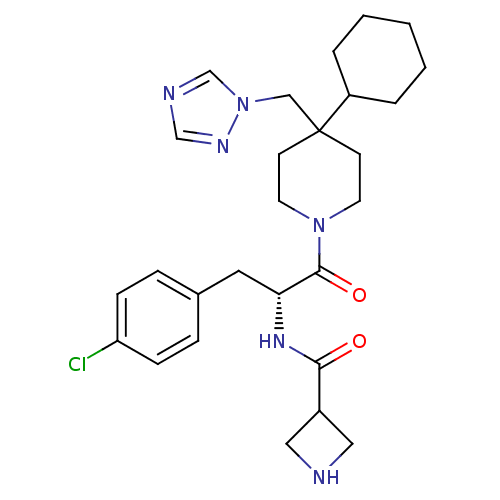 (Azetidine-3-carboxylic acid [(R)-1-(4-chloro-benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136363 (3-Amino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136356 (CHEMBL343742 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136369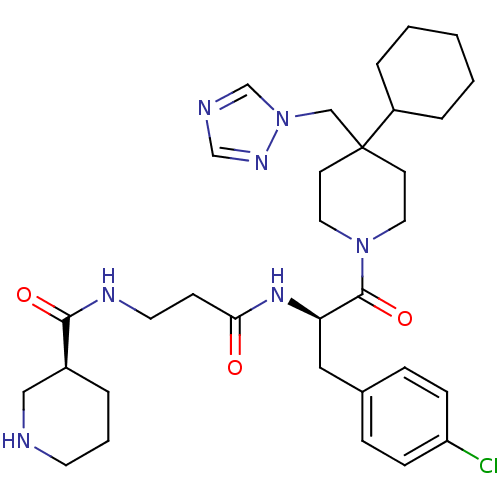 ((S)-Piperidine-3-carboxylic acid {2-[(R)-1-(4-chlo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136371 (CHEMBL343720 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136361 ((R)-Piperidine-3-carboxylic acid {2-[(R)-1-(4-chlo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136362 (3-Amino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136359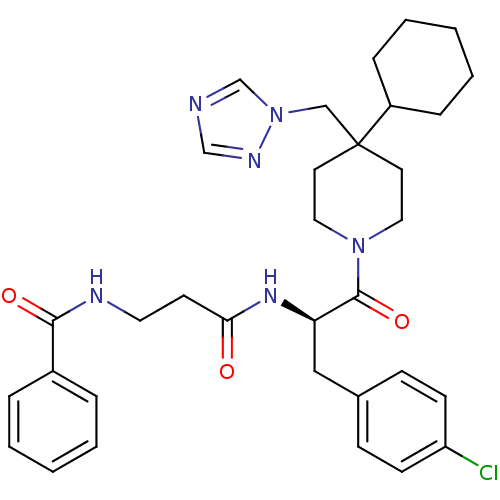 (CHEMBL136366 | N-{2-[(R)-1-(4-Chloro-benzyl)-2-(4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136373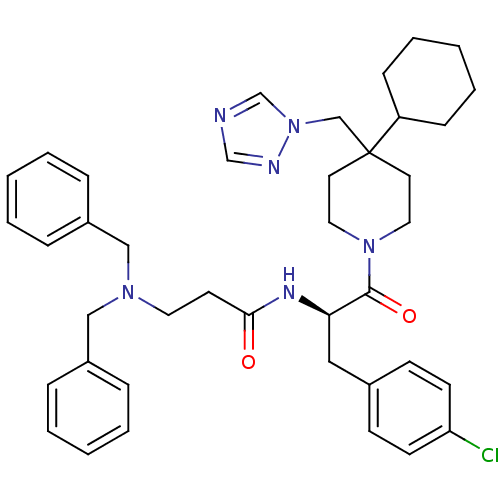 (CHEMBL340959 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136358 (CHEMBL137318 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135642 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135647 (CHEMBL328861 | N-(2-Benzylsulfanyl-phenyl)-N-(3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135642 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135642 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135638 (Benzo[b]thiophene-2-carboxylic acid (2-butyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135646 ((1R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135651 (Benzo[b]thiophene-2-carboxylic acid biphenyl-2-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135650 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135649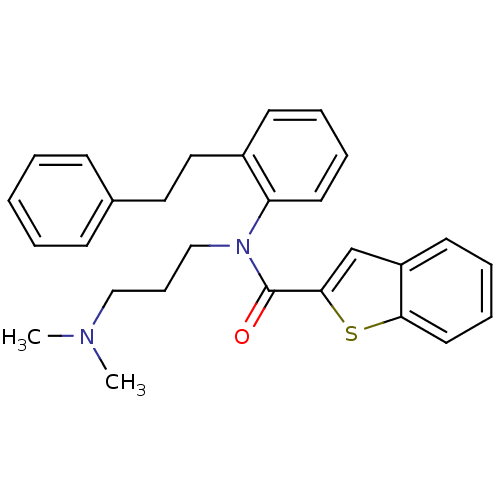 (Benzo[b]thiophene-2-carboxylic acid (3-dimethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135644 (Benzo[b]thiophene-2-carboxylic acid (2-benzoylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135645 ((E)-N-(2-Benzylsulfanyl-phenyl)-N-(3-dimethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135643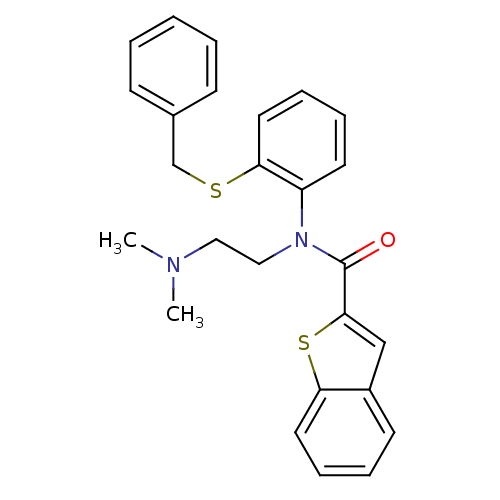 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135641 (Benzo[b]thiophene-2-carboxylic acid (2-benzyl-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135640 (3-Chloro-benzo[b]thiophene-2-carboxylic acid (2-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135639 (Benzofuran-2-carboxylic acid (2-benzylsulfanyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50135648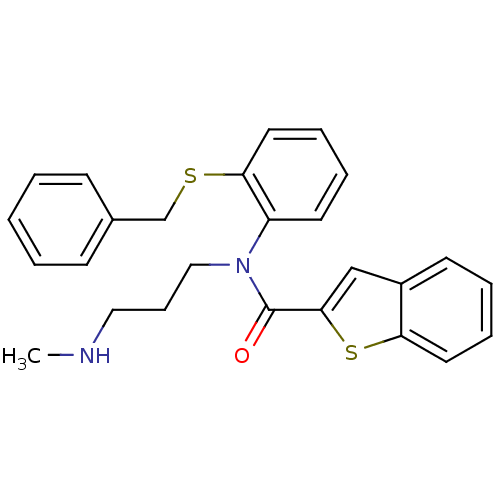 (Benzo[b]thiophene-2-carboxylic acid (2-benzylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against SMS-KAN cell membranes endogenously expressing Neuropeptide Y receptor type 2 using [125I]-PYY as radioligand | Bioorg Med Chem Lett 13: 2883-5 (2003) BindingDB Entry DOI: 10.7270/Q2F47NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136357 (CHEMBL341982 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Stimulation of adenylate cyclase in HEK293 cells expressing the human MC4R receptor was determined by measuring cAMP accumulation using the RPA559 SP... | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136365 (CHEMBL337571 | N-[(R)-1-(4-Chloro-benzyl)-2-(4-cyc...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding activity was measured using membranes of Hi5 cells expressing the human MC4R receptors | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50136355 (3-Amino-N-[(R)-1-(4-chloro-benzyl)-2-(4-cyclohexyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Stimulation of adenylate cyclase in HEK293 cells expressing the human MC4R receptor was determined by measuring cAMP accumulation using the RPA559 SP... | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Stimulation of adenylate cyclase in HEK293 cells expressing the human MC4R receptor was determined by measuring cAMP accumulation using the RPA559 SP... | Bioorg Med Chem Lett 13: 4341-4 (2003) BindingDB Entry DOI: 10.7270/Q2F18Z4D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||