

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50049391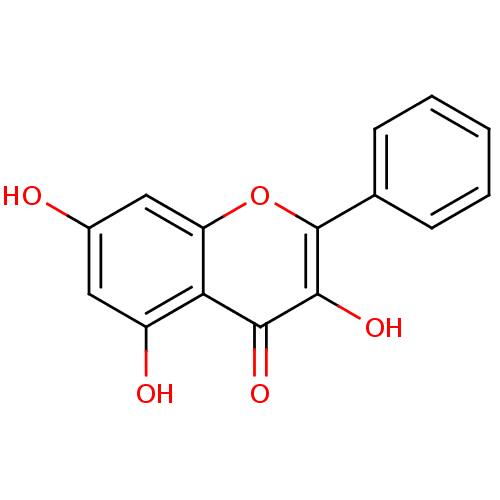 (3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells at 10 umol/L by ELISA | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells at 30 umol/L by ELISA | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7459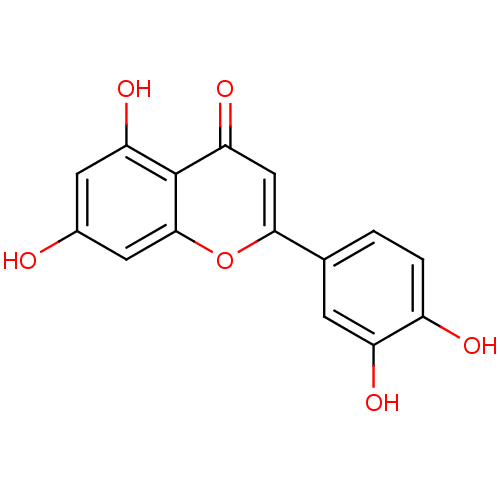 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50049391 (3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM7457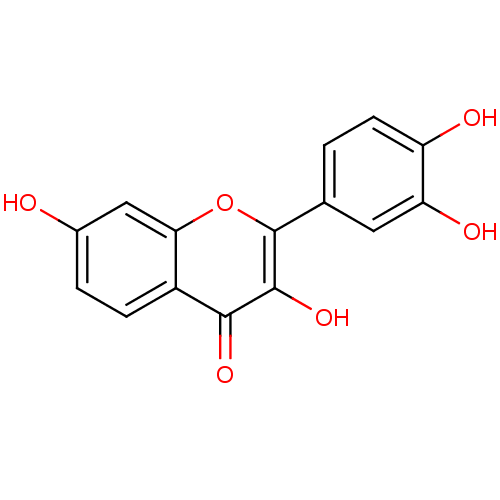 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7457 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50217942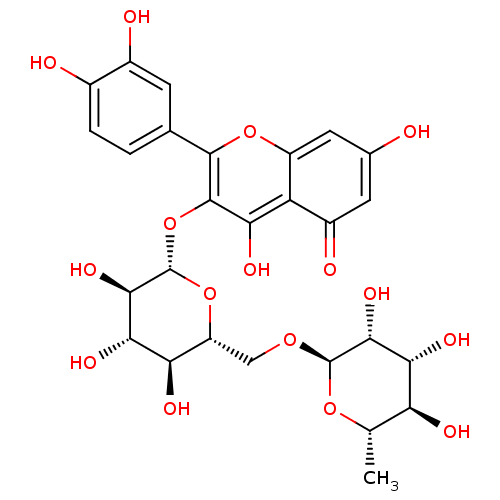 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50217942 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health Curated by ChEMBL | Assay Description Inhibition of human plasma BChE by Ellman's method | Eur J Med Chem 45: 186-92 (2010) Article DOI: 10.1016/j.ejmech.2009.09.041 BindingDB Entry DOI: 10.7270/Q2BP03Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889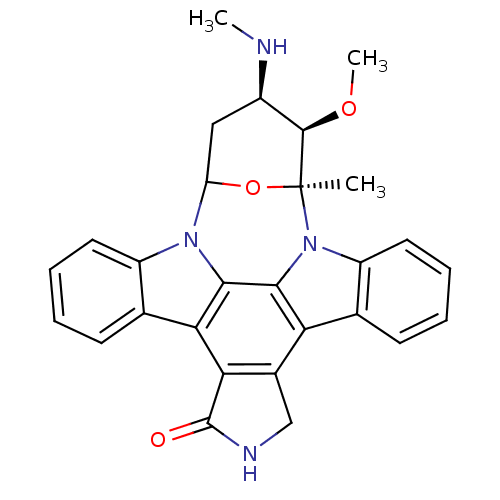 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 10 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50133496 ((2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Centre Zagreb Curated by ChEMBL | Assay Description Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 10 umol/L ATP | J Med Chem 50: 1090-100 (2007) Article DOI: 10.1021/jm0607202 BindingDB Entry DOI: 10.7270/Q2F190H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||