 Found 2925 hits with Last Name = 'armstrong' and Initial = 'rc'
Found 2925 hits with Last Name = 'armstrong' and Initial = 'rc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50400601
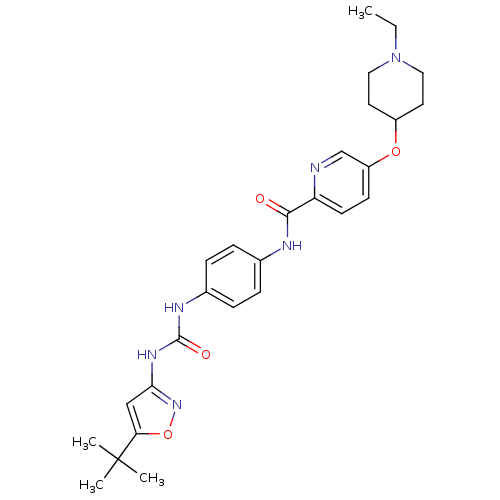
(CHEMBL2203434)Show SMILES CCN1CCC(CC1)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H34N6O4/c1-5-33-14-12-20(13-15-33)36-21-10-11-22(28-17-21)25(34)29-18-6-8-19(9-7-18)30-26(35)31-24-16-23(37-32-24)27(2,3)4/h6-11,16-17,20H,5,12-15H2,1-4H3,(H,29,34)(H2,30,31,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 phosphorylation in human MV4-11 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394785
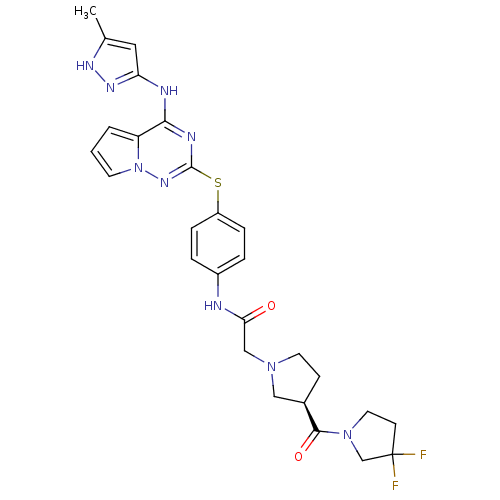
(CHEMBL2163404)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCC(F)(F)C4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H29F2N9O2S/c1-17-13-22(34-33-17)31-24-21-3-2-10-38(21)35-26(32-24)41-20-6-4-19(5-7-20)30-23(39)15-36-11-8-18(14-36)25(40)37-12-9-27(28,29)16-37/h2-7,10,13,18H,8-9,11-12,14-16H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50400602
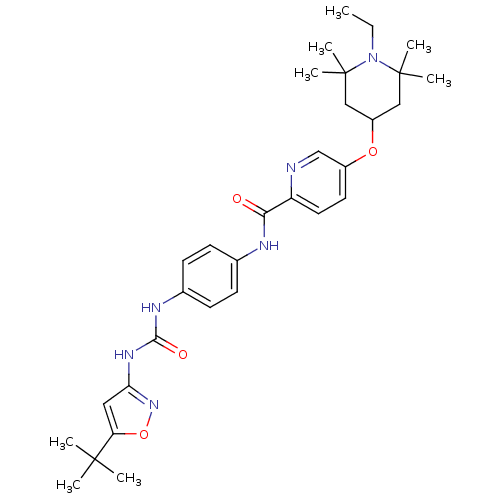
(CHEMBL2206278)Show SMILES CCN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-9-37-30(5,6)17-23(18-31(37,7)8)40-22-14-15-24(32-19-22)27(38)33-20-10-12-21(13-11-20)34-28(39)35-26-16-25(41-36-26)29(2,3)4/h10-16,19,23H,9,17-18H2,1-8H3,(H,33,38)(H2,34,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KIT phosphorylation in human H526 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50400600
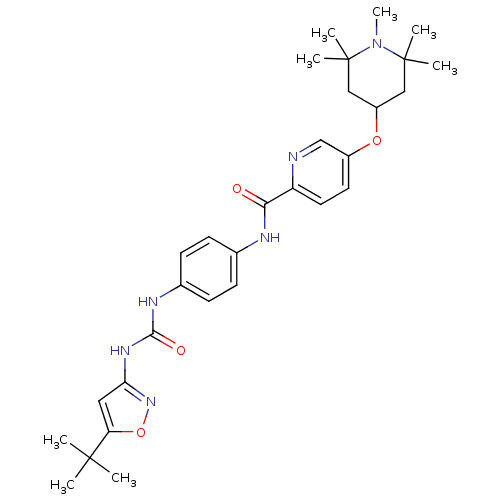
(CHEMBL2206277)Show SMILES CN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H40N6O4/c1-28(2,3)24-15-25(35-40-24)34-27(38)33-20-11-9-19(10-12-20)32-26(37)23-14-13-21(18-31-23)39-22-16-29(4,5)36(8)30(6,7)17-22/h9-15,18,22H,16-17H2,1-8H3,(H,32,37)(H2,33,34,35,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 phosphorylation in human MV4-11 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50394779
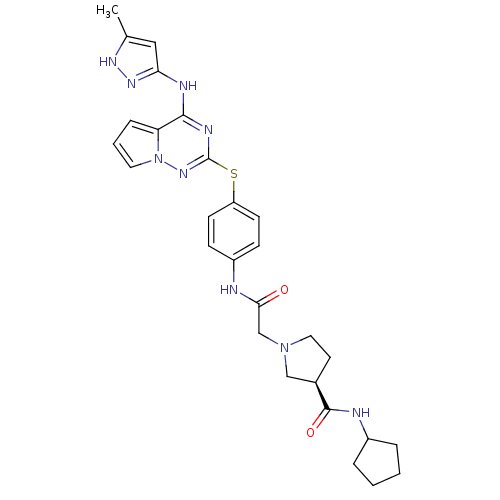
(CHEMBL2163394)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H33N9O2S/c1-18-15-24(34-33-18)31-26-23-7-4-13-37(23)35-28(32-26)40-22-10-8-21(9-11-22)29-25(38)17-36-14-12-19(16-36)27(39)30-20-5-2-3-6-20/h4,7-11,13,15,19-20H,2-3,5-6,12,14,16-17H2,1H3,(H,29,38)(H,30,39)(H2,31,32,33,34,35)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50400602

(CHEMBL2206278)Show SMILES CCN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-9-37-30(5,6)17-23(18-31(37,7)8)40-22-14-15-24(32-19-22)27(38)33-20-10-12-21(13-11-20)34-28(39)35-26-16-25(41-36-26)29(2,3)4/h10-16,19,23H,9,17-18H2,1-8H3,(H,33,38)(H2,34,35,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 phosphorylation in human MV4-11 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human AML cells isolated from relapsed acute myeloid leukemia patient by Western blotting |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060
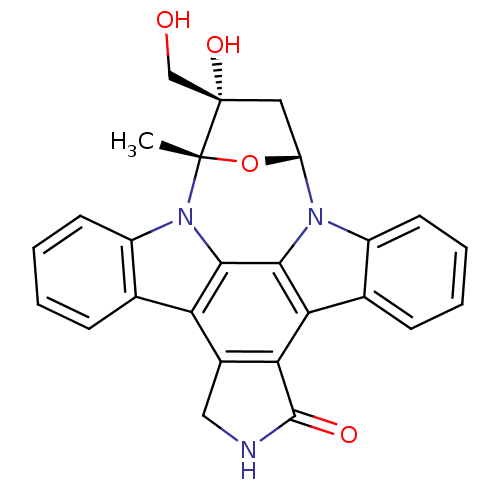
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394779

(CHEMBL2163394)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H33N9O2S/c1-18-15-24(34-33-18)31-26-23-7-4-13-37(23)35-28(32-26)40-22-10-8-21(9-11-22)29-25(38)17-36-14-12-19(16-36)27(39)30-20-5-2-3-6-20/h4,7-11,13,15,19-20H,2-3,5-6,12,14,16-17H2,1H3,(H,29,38)(H,30,39)(H2,31,32,33,34,35)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394785

(CHEMBL2163404)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCC(F)(F)C4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H29F2N9O2S/c1-17-13-22(34-33-17)31-24-21-3-2-10-38(21)35-26(32-24)41-20-6-4-19(5-7-20)30-23(39)15-36-11-8-18(14-36)25(40)37-12-9-27(28,29)16-37/h2-7,10,13,18H,8-9,11-12,14-16H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394777
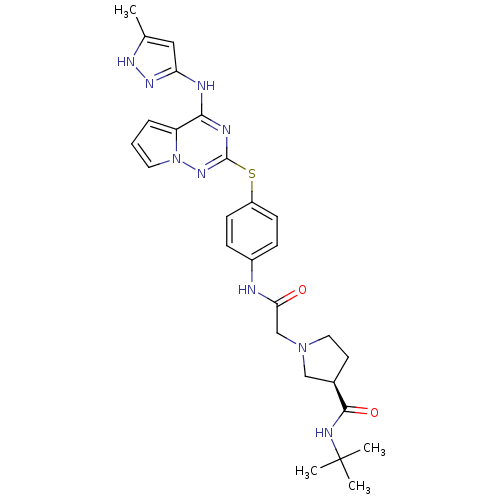
(CHEMBL2163387)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H33N9O2S/c1-17-14-22(33-32-17)29-24-21-6-5-12-36(21)34-26(30-24)39-20-9-7-19(8-10-20)28-23(37)16-35-13-11-18(15-35)25(38)31-27(2,3)4/h5-10,12,14,18H,11,13,15-16H2,1-4H3,(H,28,37)(H,31,38)(H2,29,30,32,33,34)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394798
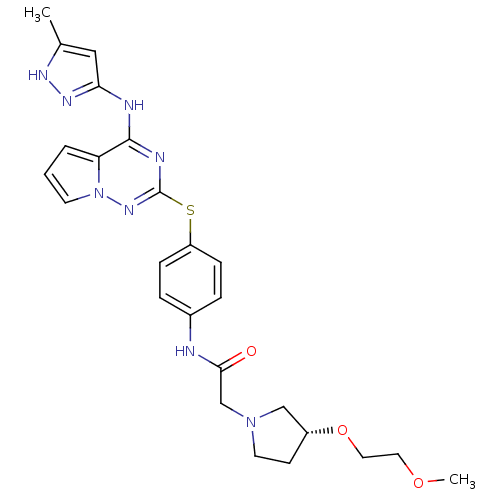
(CHEMBL2163408)Show SMILES COCCO[C@@H]1CCN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C1 |r| Show InChI InChI=1S/C25H30N8O3S/c1-17-14-22(30-29-17)27-24-21-4-3-10-33(21)31-25(28-24)37-20-7-5-18(6-8-20)26-23(34)16-32-11-9-19(15-32)36-13-12-35-2/h3-8,10,14,19H,9,11-13,15-16H2,1-2H3,(H,26,34)(H2,27,28,29,30,31)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50400601

(CHEMBL2203434)Show SMILES CCN1CCC(CC1)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H34N6O4/c1-5-33-14-12-20(13-15-33)36-21-10-11-22(28-17-21)25(34)29-18-6-8-19(9-7-18)30-26(35)31-24-16-23(37-32-24)27(2,3)4/h6-11,16-17,20H,5,12-15H2,1-4H3,(H,29,34)(H2,30,31,32,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human MG63 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394786
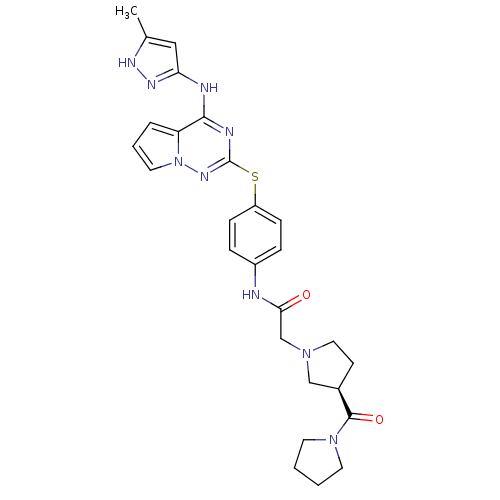
(CHEMBL2163403)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-18-15-23(32-31-18)29-25-22-5-4-13-36(22)33-27(30-25)39-21-8-6-20(7-9-21)28-24(37)17-34-14-10-19(16-34)26(38)35-11-2-3-12-35/h4-9,13,15,19H,2-3,10-12,14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50400601

(CHEMBL2203434)Show SMILES CCN1CCC(CC1)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H34N6O4/c1-5-33-14-12-20(13-15-33)36-21-10-11-22(28-17-21)25(34)29-18-6-8-19(9-7-18)30-26(35)31-24-16-23(37-32-24)27(2,3)4/h6-11,16-17,20H,5,12-15H2,1-4H3,(H,29,34)(H2,30,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of M-CSF-induced FK506 -fussed CSF1R phosphorylation expressed in HEK293 cells after 2 hrs by sandwich ELISA |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394776
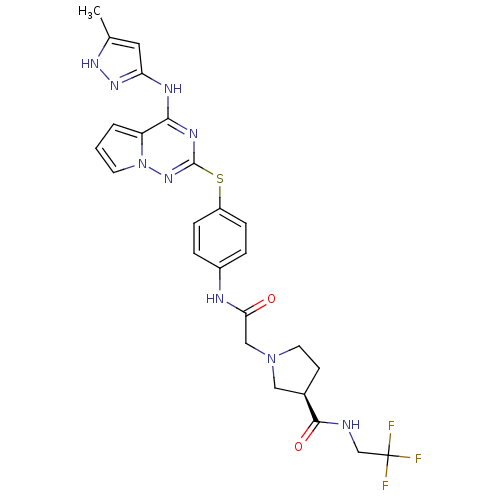
(CHEMBL2163388)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NCC(F)(F)F)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C25H26F3N9O2S/c1-15-11-20(34-33-15)31-22-19-3-2-9-37(19)35-24(32-22)40-18-6-4-17(5-7-18)30-21(38)13-36-10-8-16(12-36)23(39)29-14-25(26,27)28/h2-7,9,11,16H,8,10,12-14H2,1H3,(H,29,39)(H,30,38)(H2,31,32,33,34,35)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50400602

(CHEMBL2206278)Show SMILES CCN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-9-37-30(5,6)17-23(18-31(37,7)8)40-22-14-15-24(32-19-22)27(38)33-20-10-12-21(13-11-20)34-28(39)35-26-16-25(41-36-26)29(2,3)4/h10-16,19,23H,9,17-18H2,1-8H3,(H,33,38)(H2,34,35,36,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human MG63 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50400601

(CHEMBL2203434)Show SMILES CCN1CCC(CC1)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H34N6O4/c1-5-33-14-12-20(13-15-33)36-21-10-11-22(28-17-21)25(34)29-18-6-8-19(9-7-18)30-26(35)31-24-16-23(37-32-24)27(2,3)4/h6-11,16-17,20H,5,12-15H2,1-4H3,(H,29,34)(H2,30,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KIT phosphorylation in human H526 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50400600

(CHEMBL2206277)Show SMILES CN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H40N6O4/c1-28(2,3)24-15-25(35-40-24)34-27(38)33-20-11-9-19(10-12-20)32-26(37)23-14-13-21(18-31-23)39-22-16-29(4,5)36(8)30(6,7)17-22/h9-15,18,22H,16-17H2,1-8H3,(H,32,37)(H2,33,34,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KIT phosphorylation in human H526 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394794
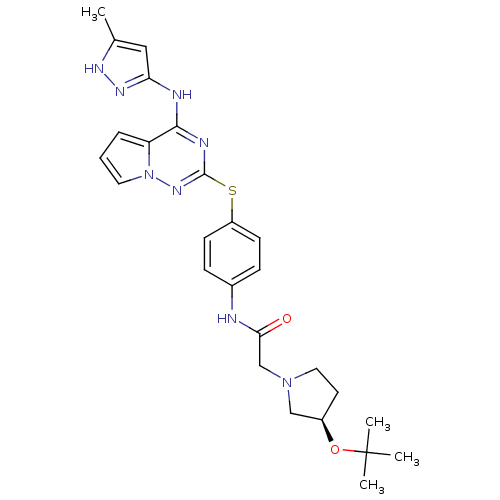
(CHEMBL2163412)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)OC(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O2S/c1-17-14-22(31-30-17)28-24-21-6-5-12-34(21)32-25(29-24)37-20-9-7-18(8-10-20)27-23(35)16-33-13-11-19(15-33)36-26(2,3)4/h5-10,12,14,19H,11,13,15-16H2,1-4H3,(H,27,35)(H2,28,29,30,31,32)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394786

(CHEMBL2163403)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-18-15-23(32-31-18)29-25-22-5-4-13-36(22)33-27(30-25)39-21-8-6-20(7-9-21)28-24(37)17-34-14-10-19(16-34)26(38)35-11-2-3-12-35/h4-9,13,15,19H,2-3,10-12,14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50400600

(CHEMBL2206277)Show SMILES CN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H40N6O4/c1-28(2,3)24-15-25(35-40-24)34-27(38)33-20-11-9-19(10-12-20)32-26(37)23-14-13-21(18-31-23)39-22-16-29(4,5)36(8)30(6,7)17-22/h9-15,18,22H,16-17H2,1-8H3,(H,32,37)(H2,33,34,35,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human MG63 cells |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394778
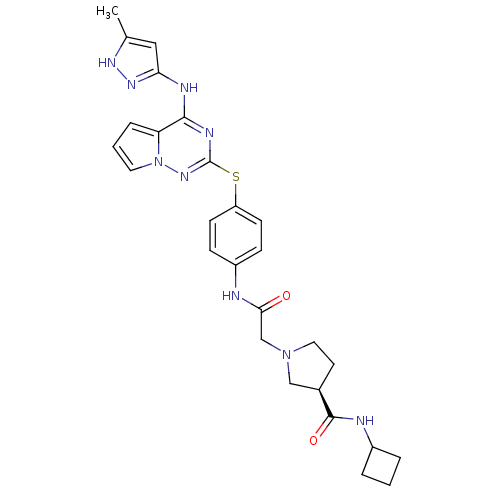
(CHEMBL2163395)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)NC4CCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-17-14-23(33-32-17)30-25-22-6-3-12-36(22)34-27(31-25)39-21-9-7-20(8-10-21)28-24(37)16-35-13-11-18(15-35)26(38)29-19-4-2-5-19/h3,6-10,12,14,18-19H,2,4-5,11,13,15-16H2,1H3,(H,28,37)(H,29,38)(H2,30,31,32,33,34)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394784
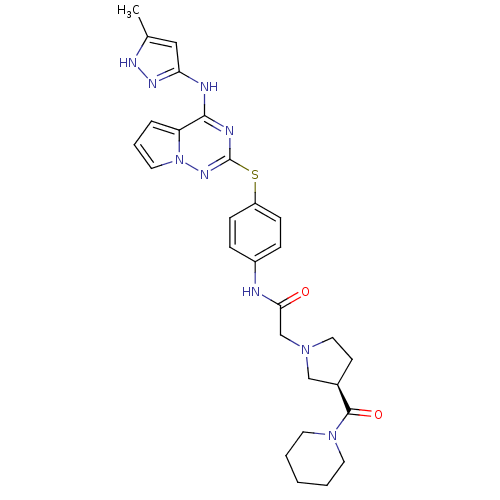
(CHEMBL2163389)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H33N9O2S/c1-19-16-24(33-32-19)30-26-23-6-5-14-37(23)34-28(31-26)40-22-9-7-21(8-10-22)29-25(38)18-35-15-11-20(17-35)27(39)36-12-3-2-4-13-36/h5-10,14,16,20H,2-4,11-13,15,17-18H2,1H3,(H,29,38)(H2,30,31,32,33,34)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50400602

(CHEMBL2206278)Show SMILES CCN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-9-37-30(5,6)17-23(18-31(37,7)8)40-22-14-15-24(32-19-22)27(38)33-20-10-12-21(13-11-20)34-28(39)35-26-16-25(41-36-26)29(2,3)4/h10-16,19,23H,9,17-18H2,1-8H3,(H,33,38)(H2,34,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of M-CSF-induced FK506 -fussed CSF1R phosphorylation expressed in HEK293 cells after 2 hrs by sandwich ELISA |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394789
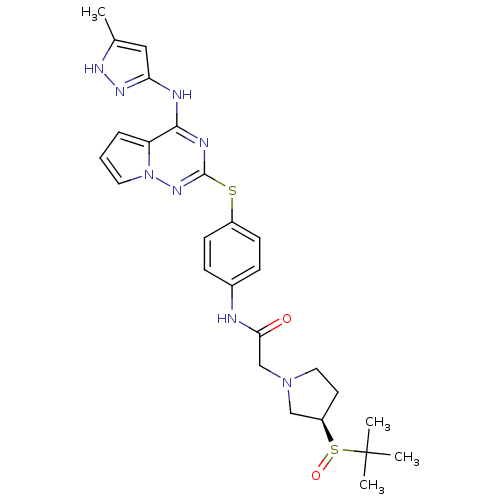
(CHEMBL2163400)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)S(=O)C(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O2S2/c1-17-14-22(31-30-17)28-24-21-6-5-12-34(21)32-25(29-24)37-19-9-7-18(8-10-19)27-23(35)16-33-13-11-20(15-33)38(36)26(2,3)4/h5-10,12,14,20H,11,13,15-16H2,1-4H3,(H,27,35)(H2,28,29,30,31,32)/t20-,38?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394799
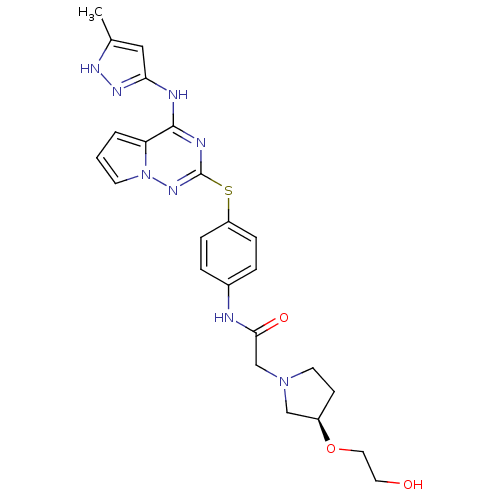
(CHEMBL2163407)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)OCCO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C24H28N8O3S/c1-16-13-21(29-28-16)26-23-20-3-2-9-32(20)30-24(27-23)36-19-6-4-17(5-7-19)25-22(34)15-31-10-8-18(14-31)35-12-11-33/h2-7,9,13,18,33H,8,10-12,14-15H2,1H3,(H,25,34)(H2,26,27,28,29,30)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352310
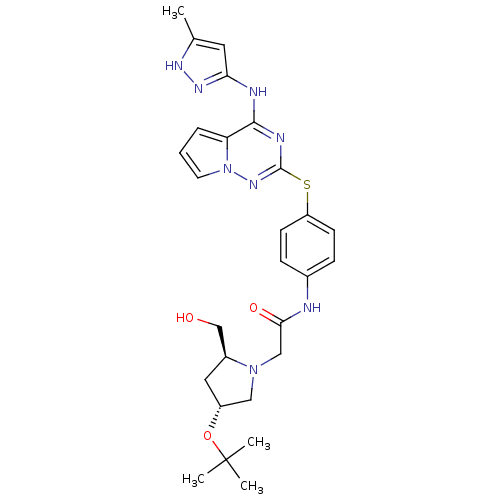
(CHEMBL1822637)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@@H](C[C@H]4CO)OC(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H34N8O3S/c1-17-12-23(32-31-17)29-25-22-6-5-11-35(22)33-26(30-25)39-21-9-7-18(8-10-21)28-24(37)15-34-14-20(13-19(34)16-36)38-27(2,3)4/h5-12,19-20,36H,13-16H2,1-4H3,(H,28,37)(H2,29,30,31,32,33)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394783
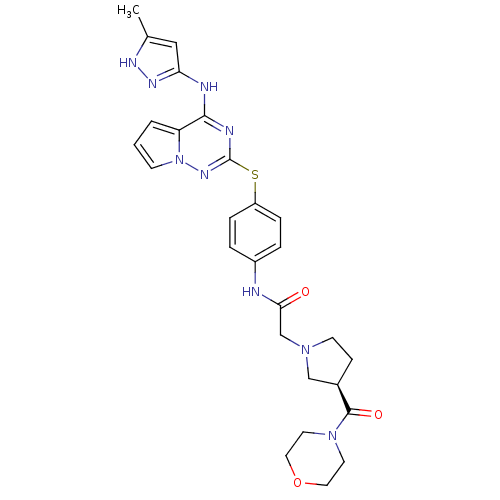
(CHEMBL2163390)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)C(=O)N4CCOCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O3S/c1-18-15-23(32-31-18)29-25-22-3-2-9-36(22)33-27(30-25)40-21-6-4-20(5-7-21)28-24(37)17-34-10-8-19(16-34)26(38)35-11-13-39-14-12-35/h2-7,9,15,19H,8,10-14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50400600

(CHEMBL2206277)Show SMILES CN1C(C)(C)CC(CC1(C)C)Oc1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H40N6O4/c1-28(2,3)24-15-25(35-40-24)34-27(38)33-20-11-9-19(10-12-20)32-26(37)23-14-13-21(18-31-23)39-22-16-29(4,5)36(8)30(6,7)17-22/h9-15,18,22H,16-17H2,1-8H3,(H,32,37)(H2,33,34,35,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of M-CSF-induced FK506 -fussed CSF1R phosphorylation expressed in HEK293 cells after 2 hrs by sandwich ELISA |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50326053
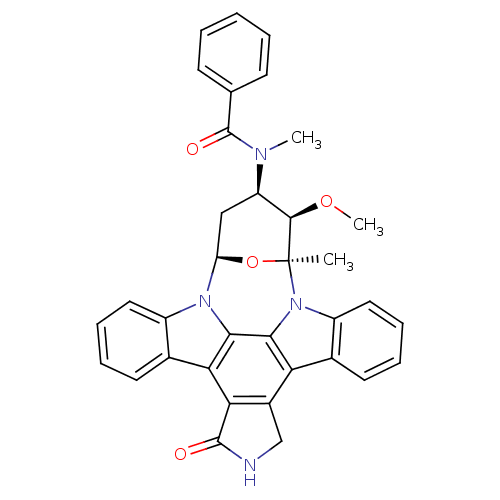
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human RS4-11 cells after 2 hrs by electrochemiluminescence assay |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352329
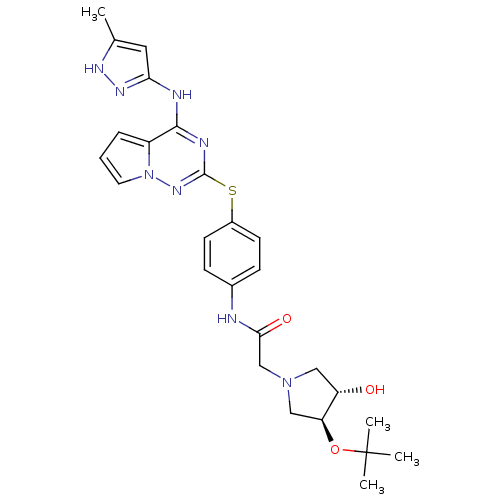
(CHEMBL1822647)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OC(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O3S/c1-16-12-22(31-30-16)28-24-19-6-5-11-34(19)32-25(29-24)38-18-9-7-17(8-10-18)27-23(36)15-33-13-20(35)21(14-33)37-26(2,3)4/h5-12,20-21,35H,13-15H2,1-4H3,(H,27,36)(H2,28,29,30,31,32)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352324
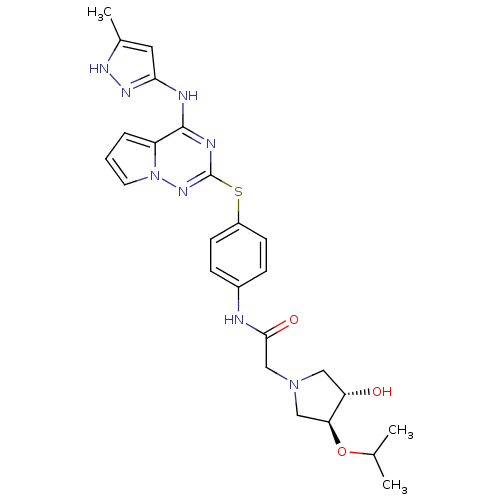
(CHEMBL1822652)Show SMILES CC(C)O[C@H]1CN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C[C@@H]1O |r| Show InChI InChI=1S/C25H30N8O3S/c1-15(2)36-21-13-32(12-20(21)34)14-23(35)26-17-6-8-18(9-7-17)37-25-28-24(19-5-4-10-33(19)31-25)27-22-11-16(3)29-30-22/h4-11,15,20-21,34H,12-14H2,1-3H3,(H,26,35)(H2,27,28,29,30,31)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352323
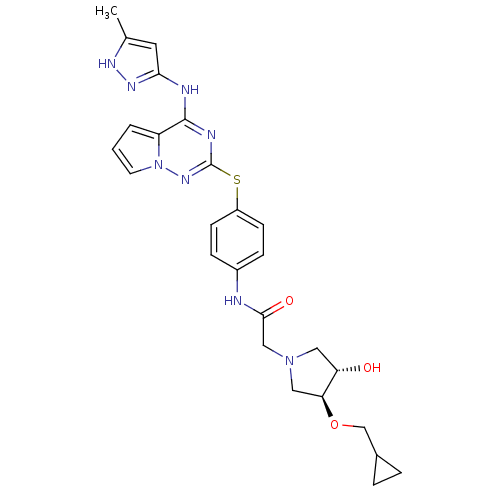
(CHEMBL1822653)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OCC4CC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H30N8O3S/c1-16-11-23(31-30-16)28-25-20-3-2-10-34(20)32-26(29-25)38-19-8-6-18(7-9-19)27-24(36)14-33-12-21(35)22(13-33)37-15-17-4-5-17/h2-3,6-11,17,21-22,35H,4-5,12-15H2,1H3,(H,27,36)(H2,28,29,30,31,32)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352322
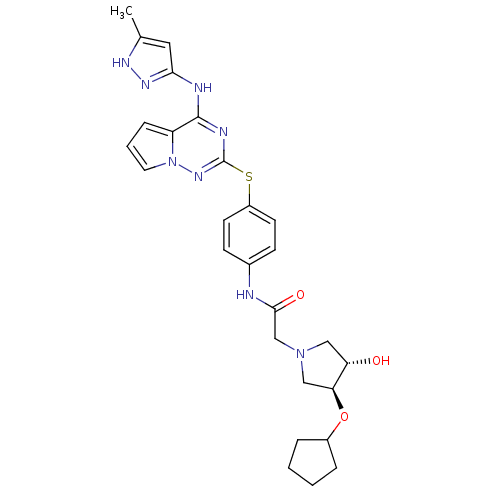
(CHEMBL1822654)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OC4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H32N8O3S/c1-17-13-24(32-31-17)29-26-21-7-4-12-35(21)33-27(30-26)39-20-10-8-18(9-11-20)28-25(37)16-34-14-22(36)23(15-34)38-19-5-2-3-6-19/h4,7-13,19,22-23,36H,2-3,5-6,14-16H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394795

(CHEMBL2163411)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)OCCCN4CCOCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C29H37N9O3S/c1-21-18-26(34-33-21)31-28-25-4-2-11-38(25)35-29(32-28)42-24-7-5-22(6-8-24)30-27(39)20-37-12-9-23(19-37)41-15-3-10-36-13-16-40-17-14-36/h2,4-8,11,18,23H,3,9-10,12-17,19-20H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352327
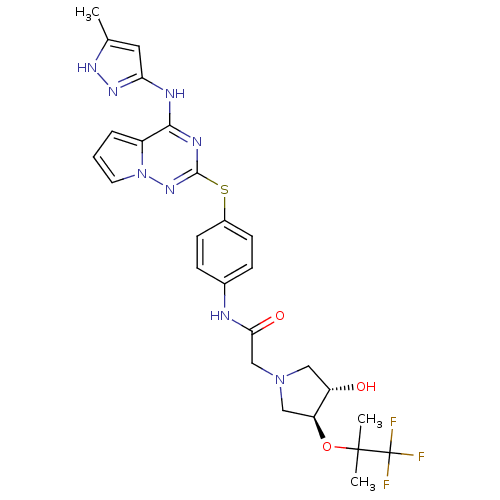
(CHEMBL1822649)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)OC(C)(C)C(F)(F)F)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H29F3N8O3S/c1-15-11-21(34-33-15)31-23-18-5-4-10-37(18)35-24(32-23)41-17-8-6-16(7-9-17)30-22(39)14-36-12-19(38)20(13-36)40-25(2,3)26(27,28)29/h4-11,19-20,38H,12-14H2,1-3H3,(H,30,39)(H2,31,32,33,34,35)/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352326
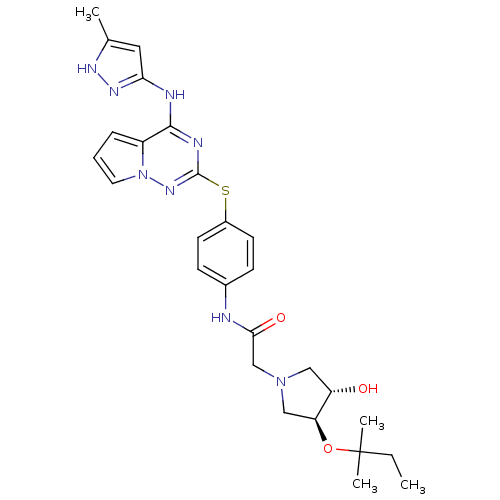
(CHEMBL1822650)Show SMILES CCC(C)(C)O[C@H]1CN(CC(=O)Nc2ccc(Sc3nc(Nc4cc(C)[nH]n4)c4cccn4n3)cc2)C[C@@H]1O |r| Show InChI InChI=1S/C27H34N8O3S/c1-5-27(3,4)38-22-15-34(14-21(22)36)16-24(37)28-18-8-10-19(11-9-18)39-26-30-25(20-7-6-12-35(20)33-26)29-23-13-17(2)31-32-23/h6-13,21-22,36H,5,14-16H2,1-4H3,(H,28,37)(H2,29,30,31,32,33)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352321

(CHEMBL1822655)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](OC(C)(C)C)C(C4)N=O)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H31N9O3S/c1-16-12-22(31-30-16)28-24-20-6-5-11-35(20)32-25(29-24)39-18-9-7-17(8-10-18)27-23(36)15-34-13-19(33-37)21(14-34)38-26(2,3)4/h5-12,19,21H,13-15H2,1-4H3,(H,27,36)(H2,28,29,30,31,32)/t19?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352307
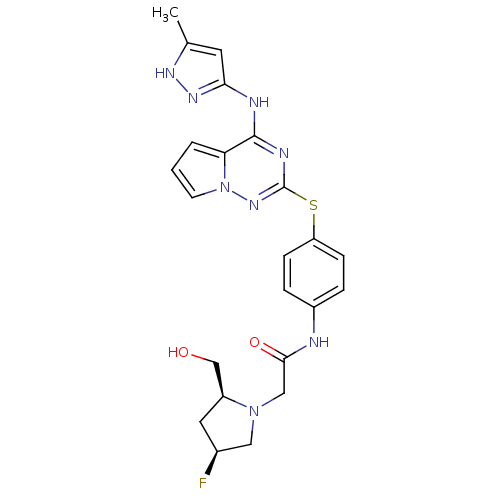
(CHEMBL1822640)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@@H](F)C[C@H]4CO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C23H25FN8O2S/c1-14-9-20(29-28-14)26-22-19-3-2-8-32(19)30-23(27-22)35-18-6-4-16(5-7-18)25-21(34)12-31-11-15(24)10-17(31)13-33/h2-9,15,17,33H,10-13H2,1H3,(H,25,34)(H2,26,27,28,29,30)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352320
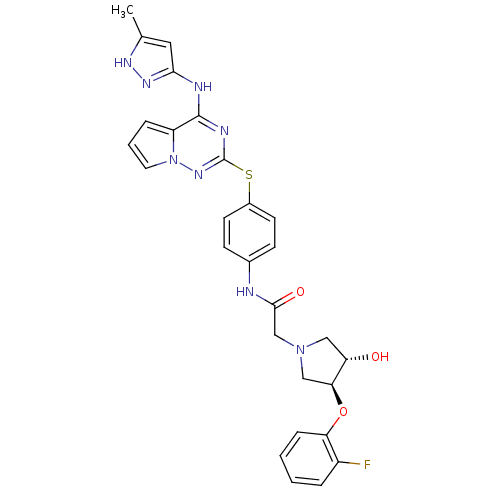
(CHEMBL1822656)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4C[C@H](O)[C@H](C4)Oc4ccccc4F)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C28H27FN8O3S/c1-17-13-25(34-33-17)31-27-21-6-4-12-37(21)35-28(32-27)41-19-10-8-18(9-11-19)30-26(39)16-36-14-22(38)24(15-36)40-23-7-3-2-5-20(23)29/h2-13,22,24,38H,14-16H2,1H3,(H,30,39)(H2,31,32,33,34,35)/t22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394788
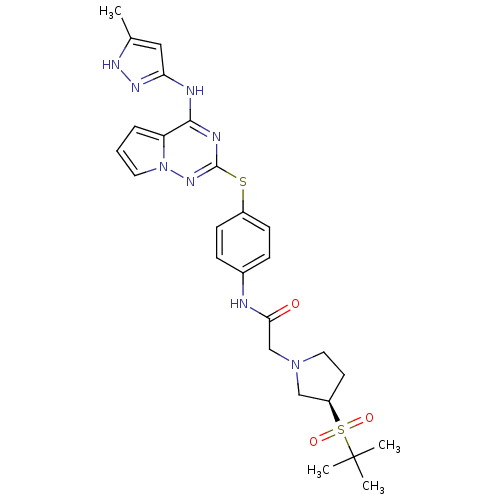
(CHEMBL2163401)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@H](C4)S(=O)(=O)C(C)(C)C)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C26H32N8O3S2/c1-17-14-22(31-30-17)28-24-21-6-5-12-34(21)32-25(29-24)38-19-9-7-18(8-10-19)27-23(35)16-33-13-11-20(15-33)39(36,37)26(2,3)4/h5-10,12,14,20H,11,13,15-16H2,1-4H3,(H,27,35)(H2,28,29,30,31,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50352309
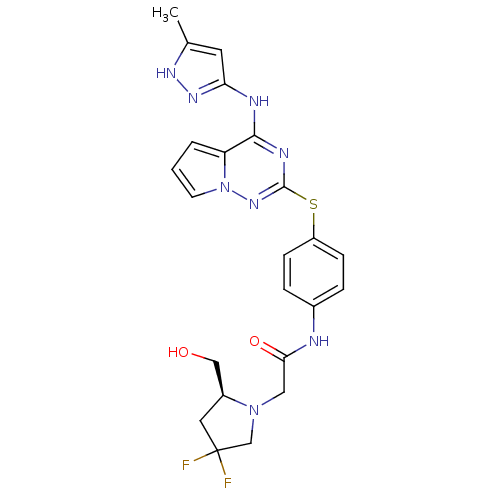
(CHEMBL1822638)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC(F)(F)C[C@H]4CO)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C23H24F2N8O2S/c1-14-9-19(30-29-14)27-21-18-3-2-8-33(18)31-22(28-21)36-17-6-4-15(5-7-17)26-20(35)11-32-13-23(24,25)10-16(32)12-34/h2-9,16,34H,10-13H2,1H3,(H,26,35)(H2,27,28,29,30,31)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase assessed as reduction in histone H3 phosphorylation in human HCT116 cells |
Bioorg Med Chem Lett 21: 5296-300 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.027
BindingDB Entry DOI: 10.7270/Q20P10D8 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Mus musculus (Mouse)) | BDBM50400609
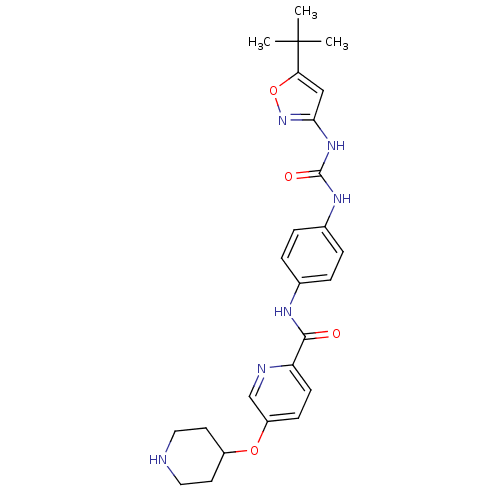
(CHEMBL2203433)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(NC(=O)c3ccc(OC4CCNCC4)cn3)cc2)no1 Show InChI InChI=1S/C25H30N6O4/c1-25(2,3)21-14-22(31-35-21)30-24(33)29-17-6-4-16(5-7-17)28-23(32)20-9-8-19(15-27-20)34-18-10-12-26-13-11-18/h4-9,14-15,18,26H,10-13H2,1-3H3,(H,28,32)(H2,29,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R in mouse M-NFS-60 cells assessed as inhibition of cell proliferation after 72 hrs by Celltiter-Blue assay |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM333480
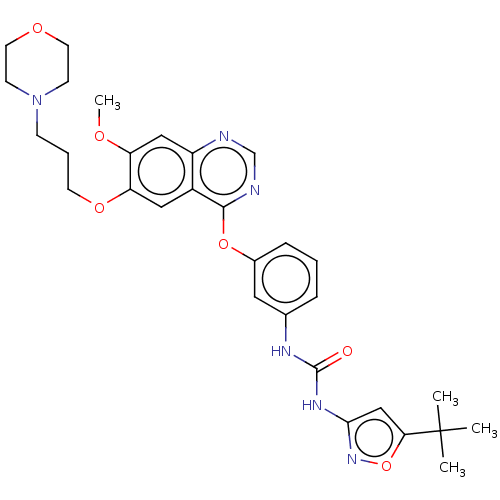
(1-(5-tert- butylisoxazol- 3-yl)-3-(3- (7-methoxy- ...)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C30H36N6O6/c1-30(2,3)26-18-27(35-42-26)34-29(37)33-20-7-5-8-21(15-20)41-28-22-16-25(24(38-4)17-23(22)31-19-32-28)40-12-6-9-36-10-13-39-14-11-36/h5,7-8,15-19H,6,9-14H2,1-4H3,(H2,33,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-dependent MEK phosphorylation in A375 cells |
Bioorg Med Chem Lett 21: 5342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.019
BindingDB Entry DOI: 10.7270/Q24T6N6Z |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Mus musculus (Mouse)) | BDBM50400614
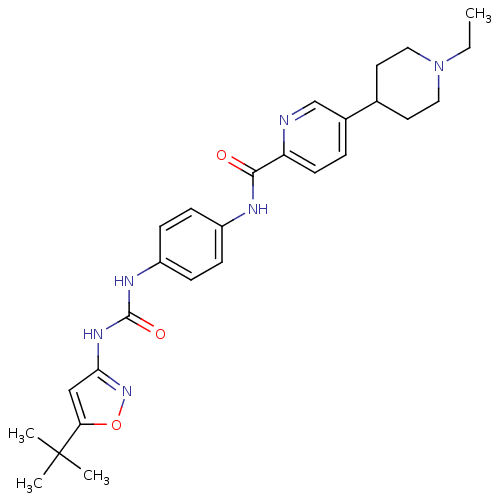
(CHEMBL2203426)Show SMILES CCN1CCC(CC1)c1ccc(nc1)C(=O)Nc1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H34N6O3/c1-5-33-14-12-18(13-15-33)19-6-11-22(28-17-19)25(34)29-20-7-9-21(10-8-20)30-26(35)31-24-16-23(36-32-24)27(2,3)4/h6-11,16-18H,5,12-15H2,1-4H3,(H,29,34)(H2,30,31,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R in mouse M-NFS-60 cells assessed as inhibition of cell proliferation after 72 hrs by Celltiter-Blue assay |
ACS Med Chem Lett 3: 997-1002 (2012)
Article DOI: 10.1021/ml300214g
BindingDB Entry DOI: 10.7270/Q22V2H9V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50394787
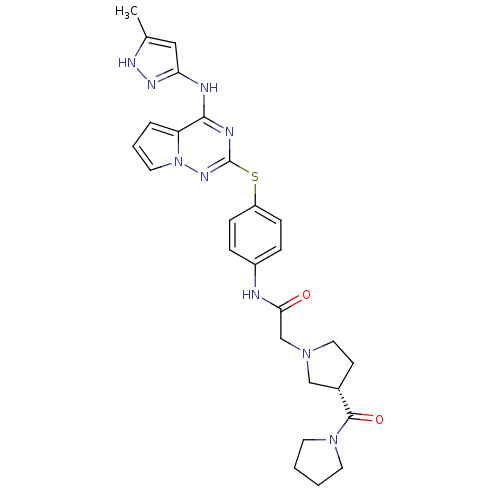
(CHEMBL2163402)Show SMILES Cc1cc(Nc2nc(Sc3ccc(NC(=O)CN4CC[C@@H](C4)C(=O)N4CCCC4)cc3)nn3cccc23)n[nH]1 |r| Show InChI InChI=1S/C27H31N9O2S/c1-18-15-23(32-31-18)29-25-22-5-4-13-36(22)33-27(30-25)39-21-8-6-20(7-9-21)28-24(37)17-34-14-10-19(16-34)26(38)35-11-2-3-12-35/h4-9,13,15,19H,2-3,10-12,14,16-17H2,1H3,(H,28,37)(H2,29,30,31,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA |
J Med Chem 55: 3250-60 (2012)
Article DOI: 10.1021/jm201702g
BindingDB Entry DOI: 10.7270/Q2W0971F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data