

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50107746 (CHEMBL3600828) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Antagonist activity against rat MCHR1 | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM142122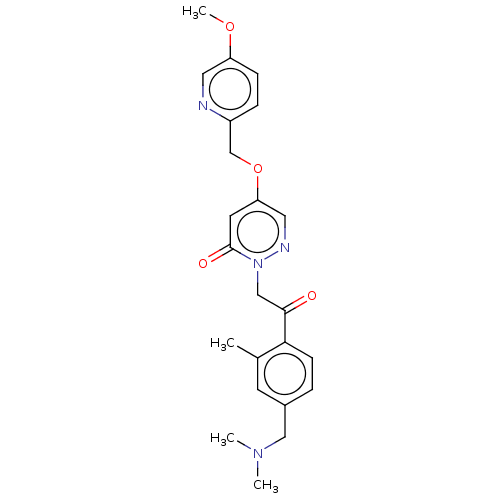 (US8933079, 149 | US8933079, 150 | US8933079, 9.1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Binding affinity to human MCH-R1 expressed in CHO/Galpha16 cells | Bioorg Med Chem Lett 25: 3275-80 (2015) Article DOI: 10.1016/j.bmcl.2015.05.065 BindingDB Entry DOI: 10.7270/Q2T43VV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4560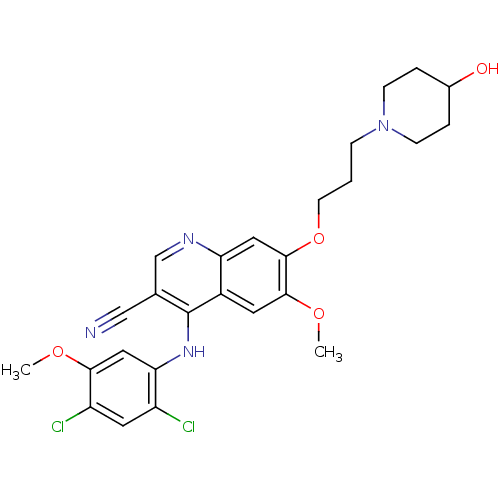 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31i |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4557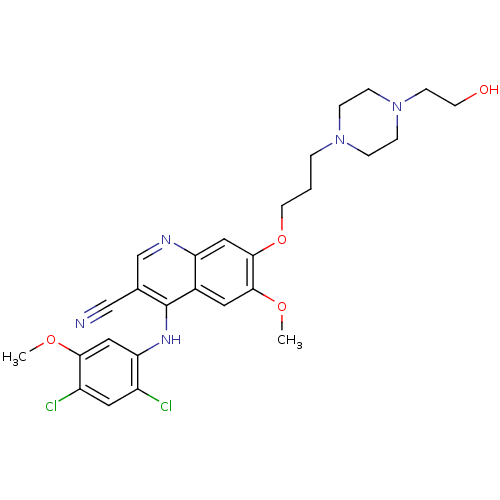 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31f |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4553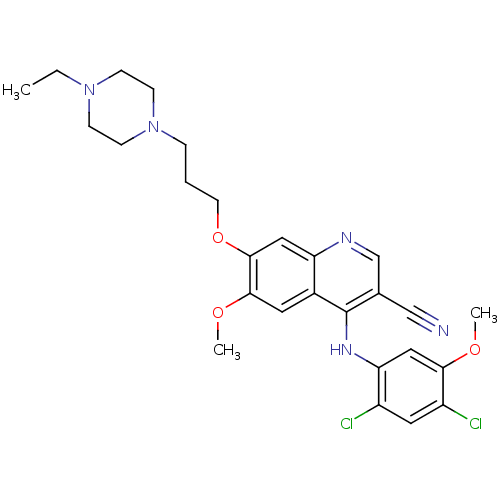 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31b |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4543 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4544 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2d | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107818 (CHEMBL3600811) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107817 (CHEMBL3600810) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4556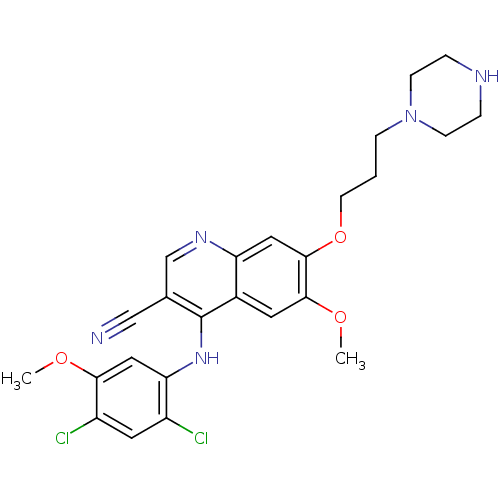 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31e |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4555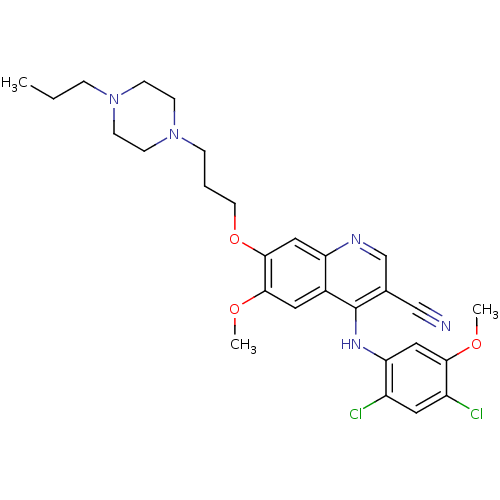 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31d |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4547 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2g | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4545 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2e | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4559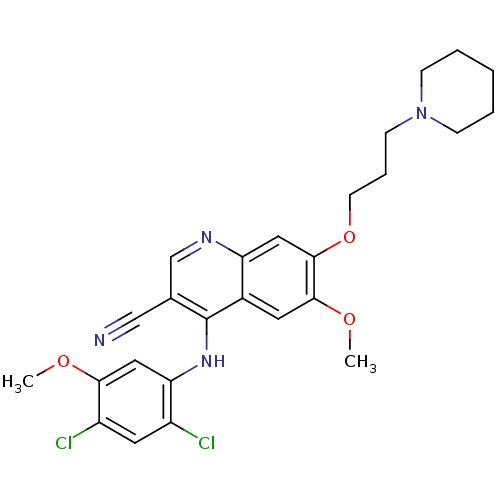 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31h |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4550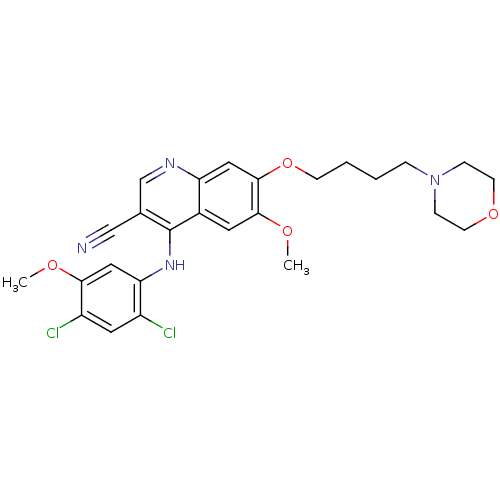 (4-Phenylamino 3-quinolinecarbonitrile deriv. 27 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4554 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31c |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4558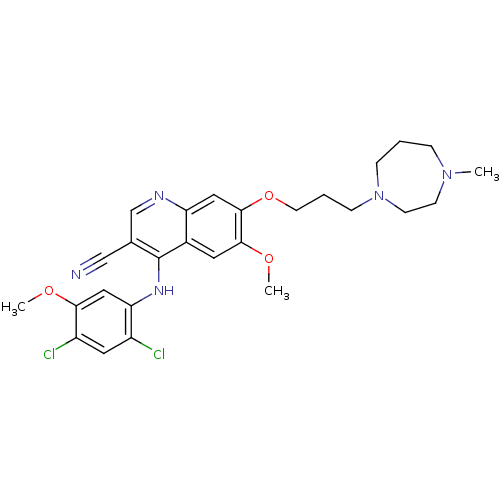 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31g |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4542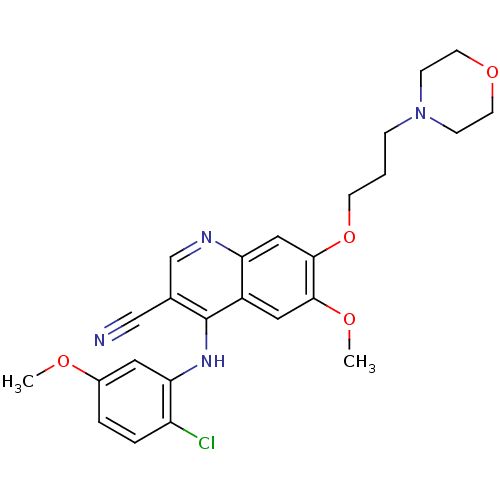 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2b | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4546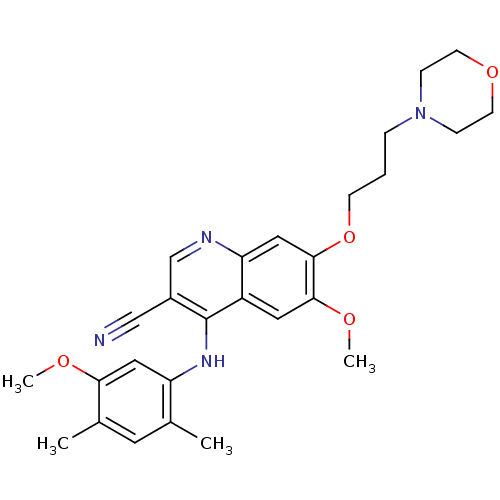 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2f | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4562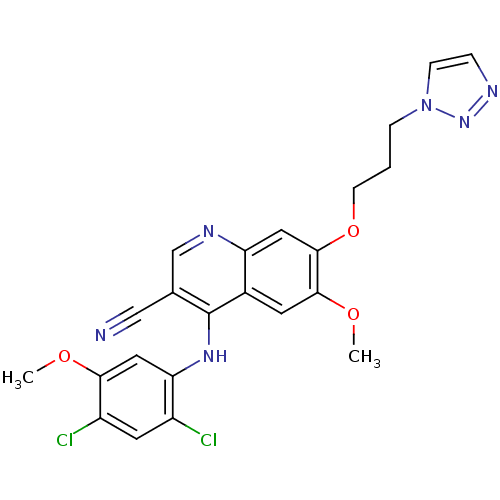 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31k |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107733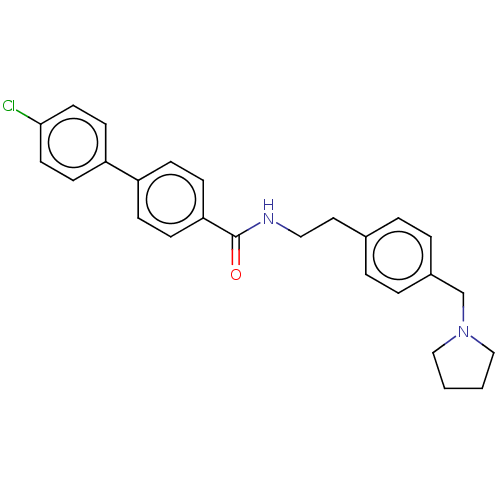 (CHEMBL3600801) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107764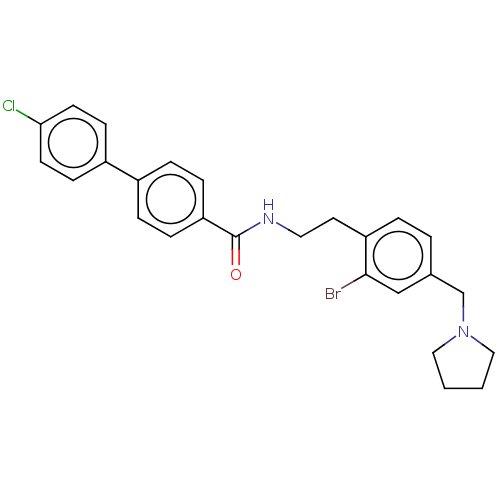 (CHEMBL3600807) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107761 (CHEMBL3600804) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107760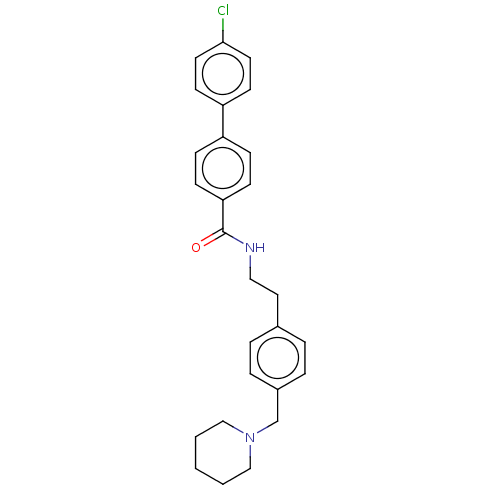 (CHEMBL3600803) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4563 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31l |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4565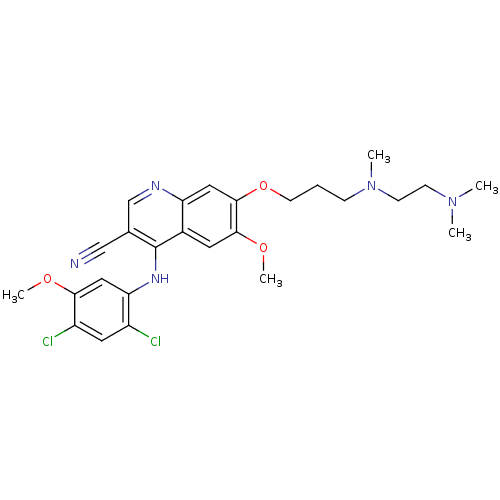 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31n |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4549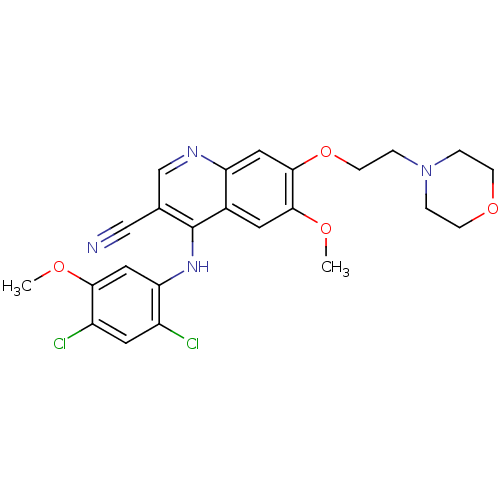 (4-Phenylamino 3-quinolinecarbonitrile deriv. 26 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4551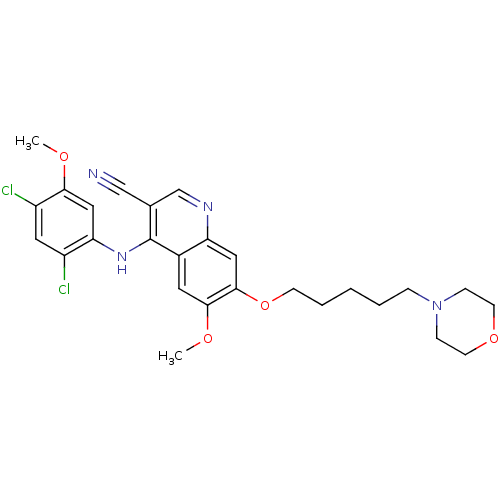 (4-Phenylamino-3-quinolinecarbonitrile deriv. 28 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4534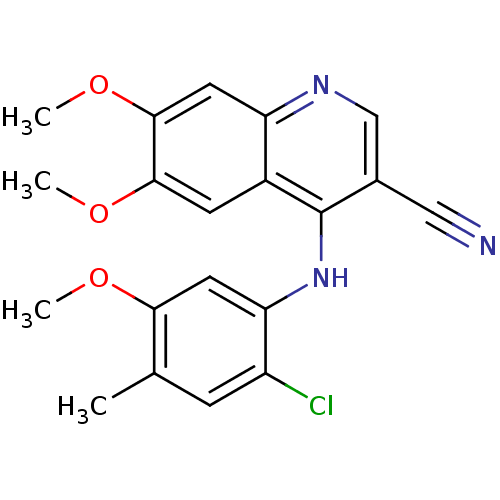 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1l | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4530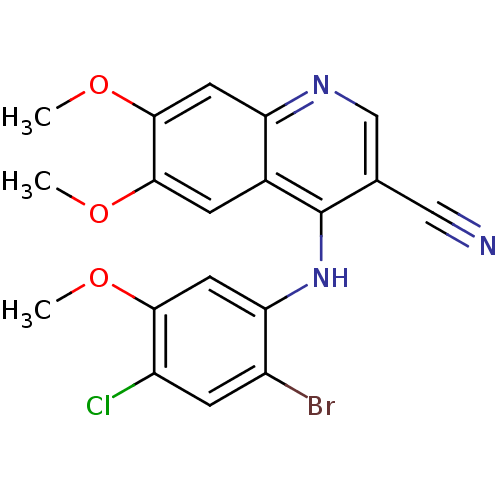 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1h | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106579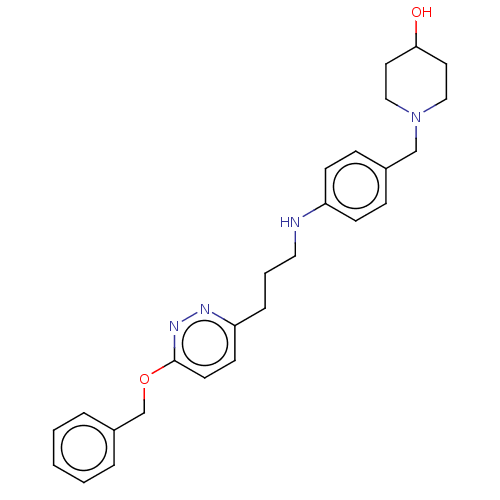 (CHEMBL3601036) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50316351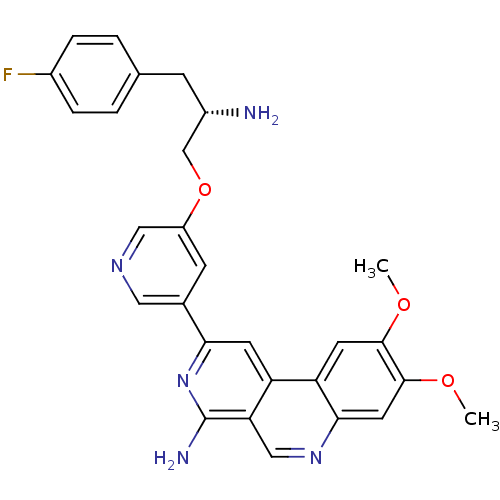 (2-(5-{[(2S)-2-Amino-3-(4-fluorophenyl)propyl]oxy}p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human GST-tagged-PDK1 expressed in HEK293 cells | Eur J Med Chem 45: 1379-86 (2010) Article DOI: 10.1016/j.ejmech.2009.12.036 BindingDB Entry DOI: 10.7270/Q2HD7VT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107735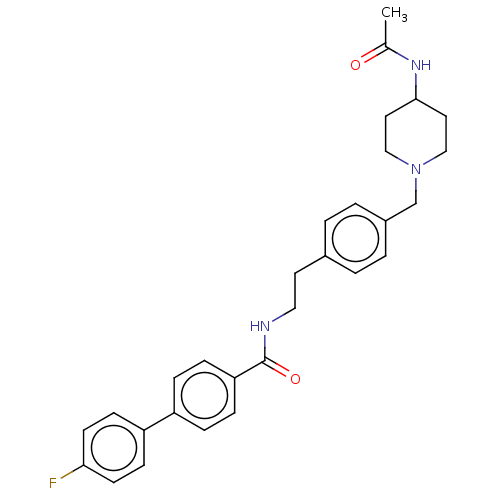 (CHEMBL3600815) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106487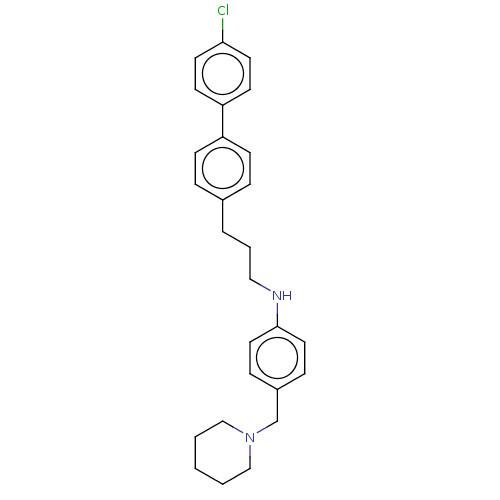 (CHEMBL3600970) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4564 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31m |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50316346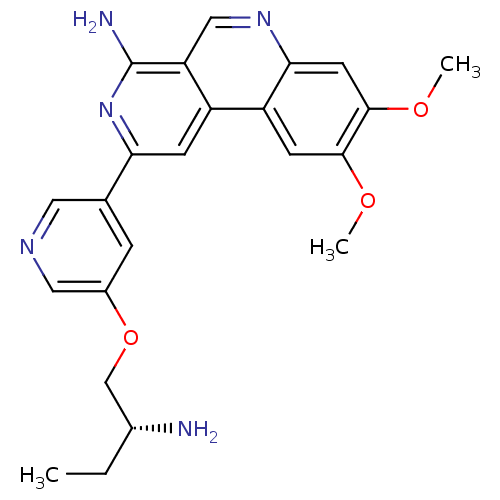 (2-(5-{[(2R)-2-Aminobutyl]oxy}pyridin-3-yl)-8,9-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human GST-tagged-PDK1 expressed in HEK293 cells | Eur J Med Chem 45: 1379-86 (2010) Article DOI: 10.1016/j.ejmech.2009.12.036 BindingDB Entry DOI: 10.7270/Q2HD7VT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50316344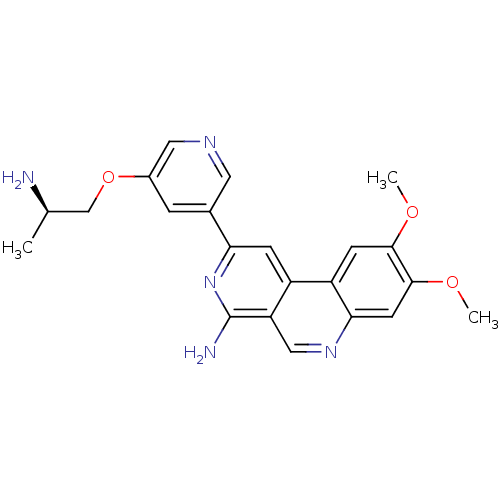 (2-(5-{[(2R)-2-Aminopropyl]oxy}pyridin-3-yl)-8,9-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human GST-tagged-PDK1 expressed in HEK293 cells | Eur J Med Chem 45: 1379-86 (2010) Article DOI: 10.1016/j.ejmech.2009.12.036 BindingDB Entry DOI: 10.7270/Q2HD7VT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107820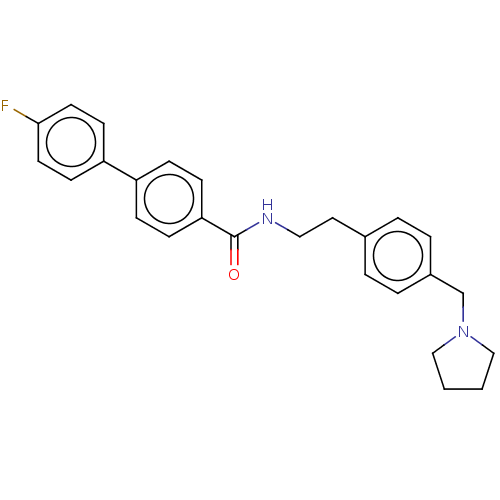 (CHEMBL3600812) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4522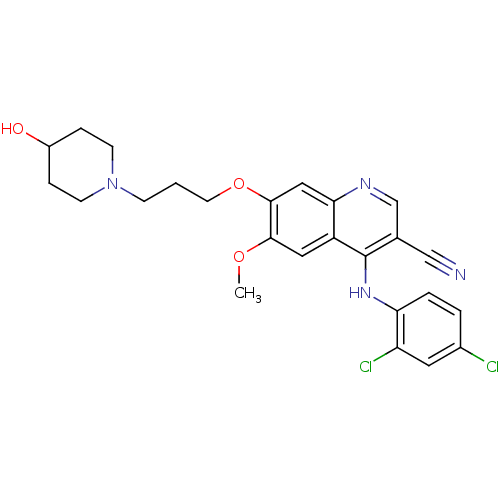 (4-Phenylamino-3-quinolinecarbonitrile deriv. 27 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4520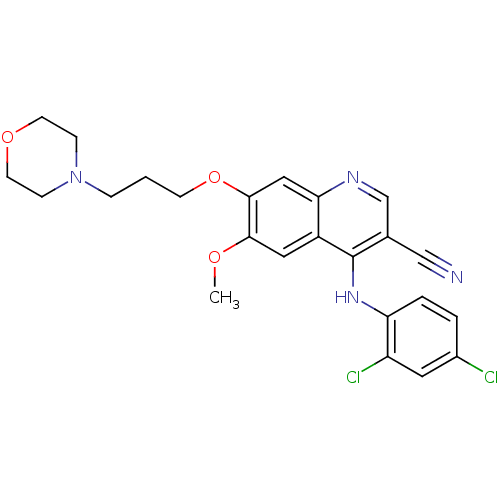 (4-Phenylamino-3-quinolinecarbonitrile deriv. 25 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4520 (4-Phenylamino-3-quinolinecarbonitrile deriv. 25 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106522 (CHEMBL3600978) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50106583 (CHEMBL3601040) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3270-4 (2015) Article DOI: 10.1016/j.bmcl.2015.05.074 BindingDB Entry DOI: 10.7270/Q2MK6FN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107751 (CHEMBL3601023) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107749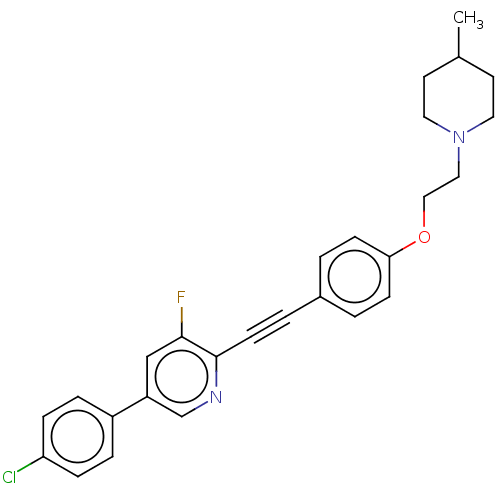 (CHEMBL3601021) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4531 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1i | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4525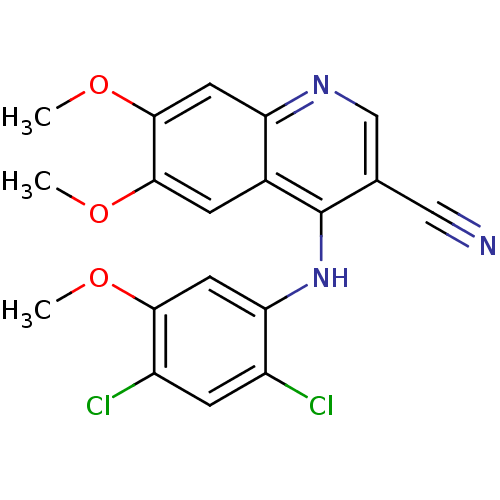 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4536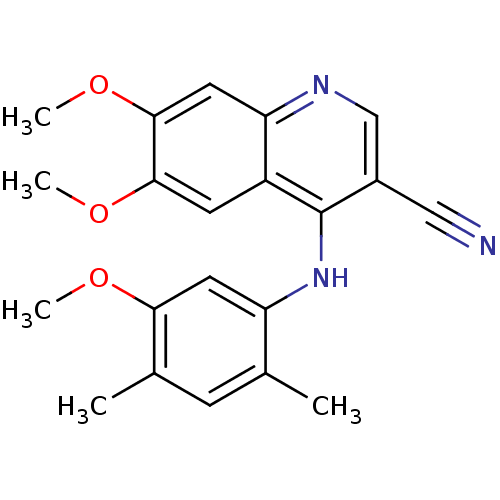 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1n | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50107754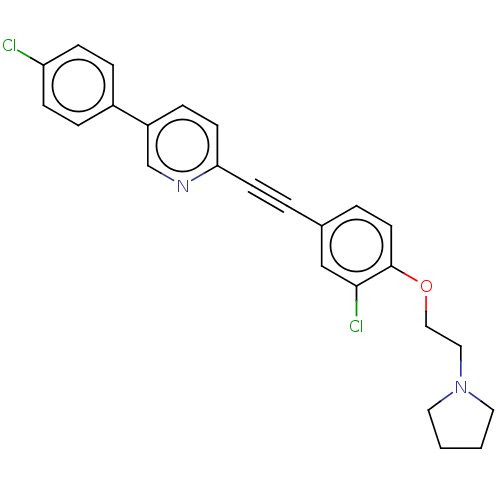 (CHEMBL3601017) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO/Galpha16 cell membranes by scintillation counting method | Bioorg Med Chem Lett 25: 3264-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.077 BindingDB Entry DOI: 10.7270/Q25D8TMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 442 total ) | Next | Last >> |