 Found 1397 hits with Last Name = 'artis' and Initial = 'dr'
Found 1397 hits with Last Name = 'artis' and Initial = 'dr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14714
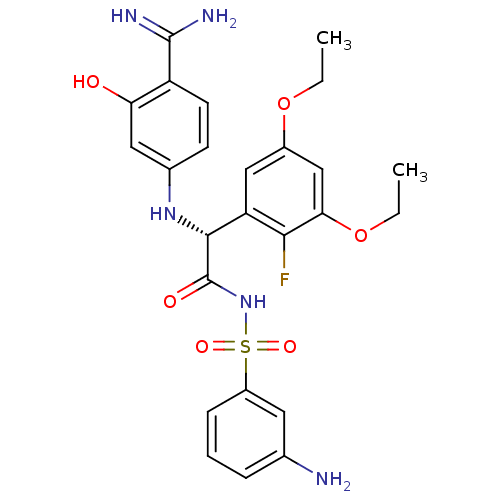
((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 87 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 114 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.40E+3 | >-30.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.50E+3 | >-30.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.90E+3 | >-30.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.00E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M]
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060228

((5S,8R,13R,15aS)-8-{[(2R,3S)-1-Acetyl-3-(4-hydroxy...)Show SMILES CC(=O)N1CC[C@H]([C@@H]1C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CO)NC1=O)C(O)=O)c1ccc(O)cc1 Show InChI InChI=1S/C27H35N5O9S2/c1-14(34)31-10-8-17(15-4-6-16(35)7-5-15)22(31)25(38)29-19-12-42-43-13-20(27(40)41)30-24(37)21-3-2-9-32(21)26(39)18(11-33)28-23(19)36/h4-7,17-22,33,35H,2-3,8-13H2,1H3,(H,28,36)(H,29,38)(H,30,37)(H,40,41)/t17-,18-,19-,20-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M]
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352624
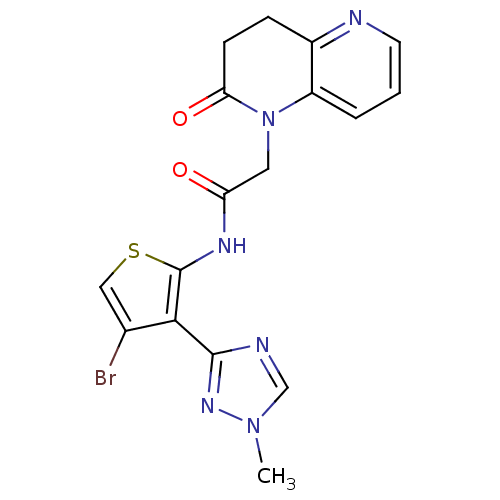
(CHEMBL1822305 | US9796706, Compound 139)Show SMILES Cn1cnc(n1)-c1c(Br)csc1NC(=O)CN1C(=O)CCc2ncccc12 Show InChI InChI=1S/C17H15BrN6O2S/c1-23-9-20-16(22-23)15-10(18)8-27-17(15)21-13(25)7-24-12-3-2-6-19-11(12)4-5-14(24)26/h2-3,6,8-9H,4-5,7H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352621
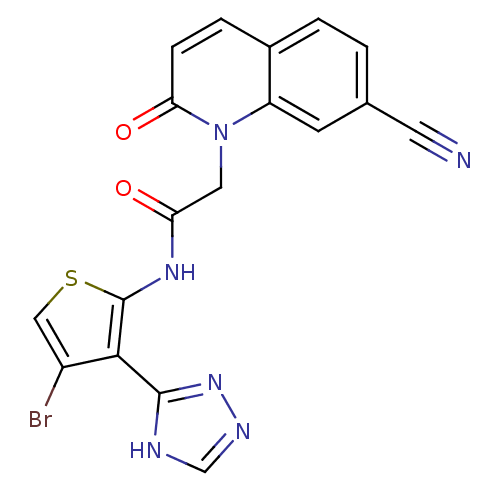
(CHEMBL1822152)Show SMILES Brc1csc(NC(=O)Cn2c3cc(ccc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-5-10(6-20)1-2-11(13)3-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060185
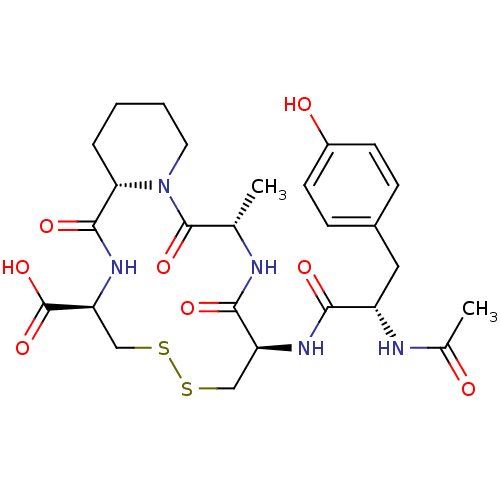
((6S,9R,14R,16aS)-9-[(S)-2-Acetylamino-3-(4-hydroxy...)Show SMILES C[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]2CCCCN2C1=O)C(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O Show InChI InChI=1S/C26H35N5O8S2/c1-14-25(37)31-10-4-3-5-21(31)24(36)30-20(26(38)39)13-41-40-12-19(23(35)27-14)29-22(34)18(28-15(2)32)11-16-6-8-17(33)9-7-16/h6-9,14,18-21,33H,3-5,10-13H2,1-2H3,(H,27,35)(H,28,32)(H,29,34)(H,30,36)(H,38,39)/t14-,18-,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438363
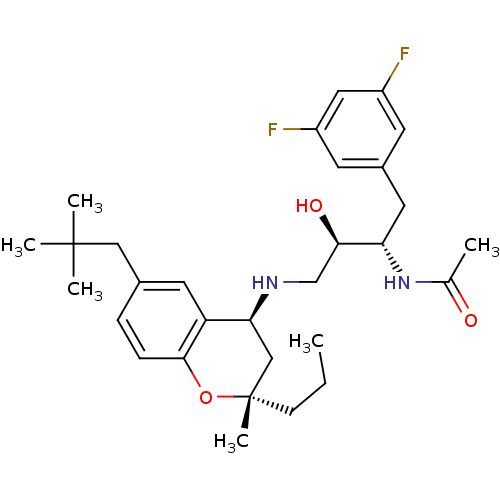
(CHEMBL2408751)Show SMILES CCC[C@@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352620
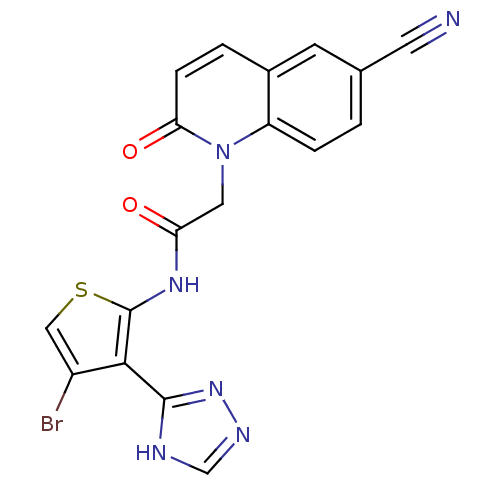
(CHEMBL1822151)Show SMILES Brc1csc(NC(=O)Cn2c3ccc(cc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-3-1-10(6-20)5-11(13)2-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM16016
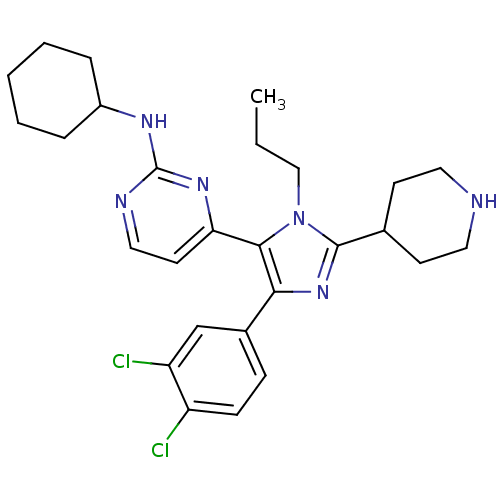
(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258304

(US9493485, 9-145)Show SMILES C[C@]1(CC2(CCC2)SC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:10| Show InChI InChI=1S/C20H20ClFN4OS/c1-19(11-20(7-2-8-20)28-18(23)26-19)14-9-13(4-5-15(14)22)25-17(27)16-6-3-12(21)10-24-16/h3-6,9-10H,2,7-8,11H2,1H3,(H2,23,26)(H,25,27)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds are also assessed for BACE1 and Cathepsin D activity using an FP Assay. Compounds to be assessed (e.g. compounds as described in the above ... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258329

(US9493485, 13-182)Show SMILES COc1cccnc1Nc1ccc(F)c(c1)[C@]1(C)CC2(CCOCC2)SC(N)=N1 |r,c:30| Show InChI InChI=1S/C21H25FN4O2S/c1-20(13-21(29-19(23)26-20)7-10-28-11-8-21)15-12-14(5-6-16(15)22)25-18-17(27-2)4-3-9-24-18/h3-6,9,12H,7-8,10-11,13H2,1-2H3,(H2,23,26)(H,24,25)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
BACE1 activity can be assessed in an AlphaScreenŽ assay. Compounds to be assessed (e.g. compounds as described in the above examples) are serially di... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060226
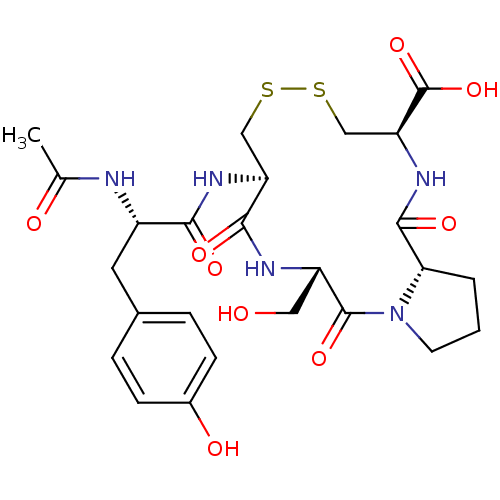
((5S,8R,13R,15aS)-8-[(S)-2-Acetylamino-3-(4-hydroxy...)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CO)NC1=O)C(O)=O Show InChI InChI=1S/C25H33N5O9S2/c1-13(32)26-16(9-14-4-6-15(33)7-5-14)21(34)28-18-11-40-41-12-19(25(38)39)29-23(36)20-3-2-8-30(20)24(37)17(10-31)27-22(18)35/h4-7,16-20,31,33H,2-3,8-12H2,1H3,(H,26,32)(H,27,35)(H,28,34)(H,29,36)(H,38,39)/t16-,17-,18-,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352621

(CHEMBL1822152)Show SMILES Brc1csc(NC(=O)Cn2c3cc(ccc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-5-10(6-20)1-2-11(13)3-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352620

(CHEMBL1822151)Show SMILES Brc1csc(NC(=O)Cn2c3ccc(cc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-3-1-10(6-20)5-11(13)2-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352628
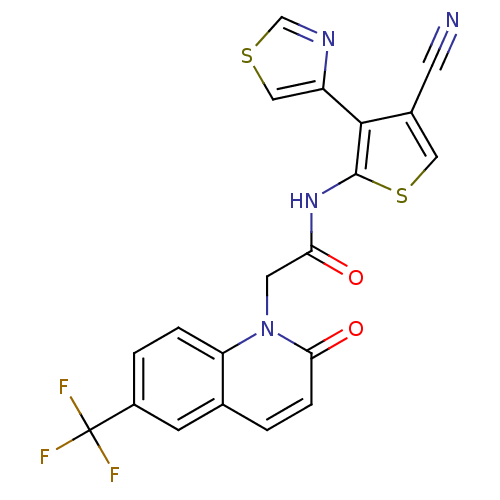
(CHEMBL1822309 | US9796706, Compound 136)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(C#N)c3-c3cscn3)c(=O)ccc2c1 Show InChI InChI=1S/C20H11F3N4O2S2/c21-20(22,23)13-2-3-15-11(5-13)1-4-17(29)27(15)7-16(28)26-19-18(12(6-24)8-31-19)14-9-30-10-25-14/h1-5,8-10H,7H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438362
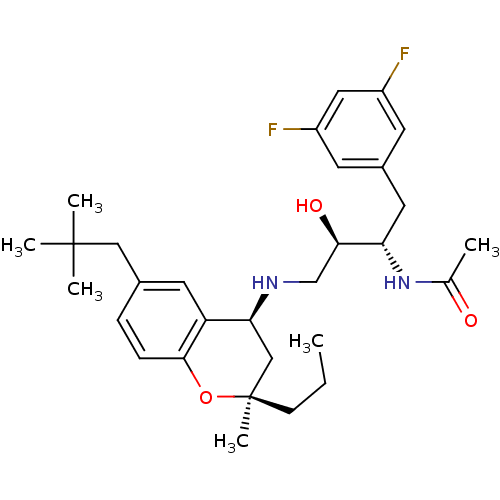
(CHEMBL2408752)Show SMILES CCC[C@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352615
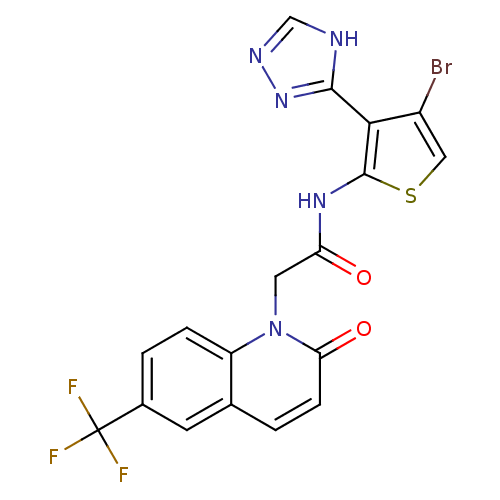
(CHEMBL1822146)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O2S/c19-11-7-30-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352618
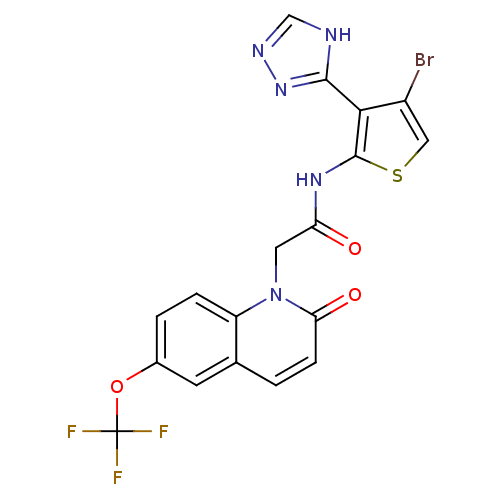
(CHEMBL1822149)Show SMILES FC(F)(F)Oc1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O3S/c19-11-7-31-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(30-18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase [531-875]
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon
| Assay Description
Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... |
Structure 12: 2233-47 (2004)
Article DOI: 10.1016/j.str.2004.10.004
BindingDB Entry DOI: 10.7270/Q25B00Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060183
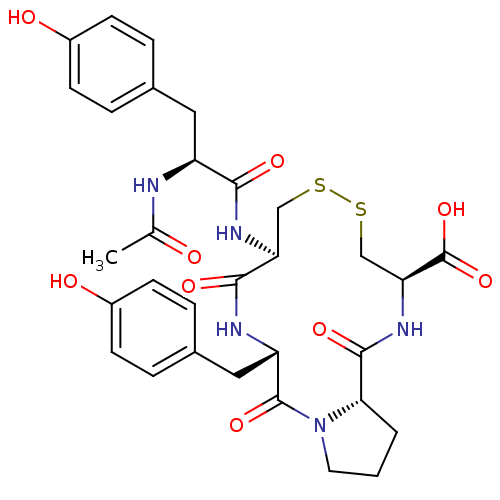
((5S,8R,13R,15aS)-8-[(S)-2-Acetylamino-3-(4-hydroxy...)Show SMILES CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(O)=O Show InChI InChI=1S/C31H37N5O9S2/c1-17(37)32-22(13-18-4-8-20(38)9-5-18)27(40)34-24-15-46-47-16-25(31(44)45)35-29(42)26-3-2-12-36(26)30(43)23(33-28(24)41)14-19-6-10-21(39)11-7-19/h4-11,22-26,38-39H,2-3,12-16H2,1H3,(H,32,37)(H,33,41)(H,34,40)(H,35,42)(H,44,45)/t22-,23-,24-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50108219

((5S,8R,13R)-8-[(S)-2-Acetylamino-3-(4-hydroxy-phen...)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CSSC[C@H](NC2=O)C(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O Show InChI InChI=1S/C26H33N5O10S2/c1-13(32)27-16(9-14-4-6-15(33)7-5-14)22(36)29-18-11-42-43-12-19(26(40)41)30-24(38)20-3-2-8-31(20)25(39)17(10-21(34)35)28-23(18)37/h4-7,16-20,33H,2-3,8-12H2,1H3,(H,27,32)(H,28,37)(H,29,36)(H,30,38)(H,34,35)(H,40,41)/t16-,17-,18-,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352614
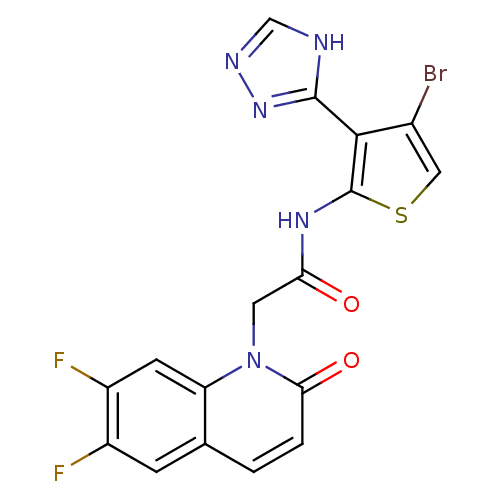
(CHEMBL1822145)Show SMILES Fc1cc2ccc(=O)n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c2cc1F Show InChI InChI=1S/C17H10BrF2N5O2S/c18-9-6-28-17(15(9)16-21-7-22-24-16)23-13(26)5-25-12-4-11(20)10(19)3-8(12)1-2-14(25)27/h1-4,6-7H,5H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352613
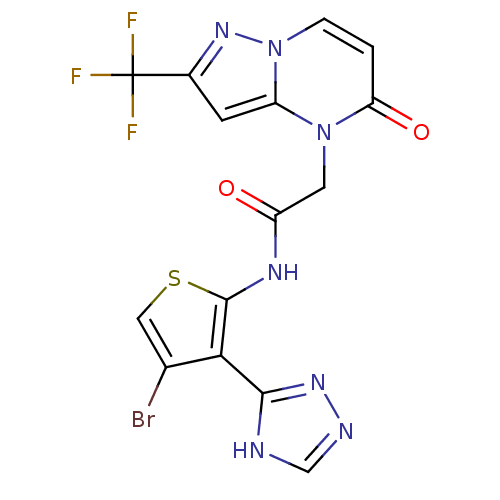
(CHEMBL1822144)Show SMILES FC(F)(F)c1cc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccn2n1 Show InChI InChI=1S/C15H9BrF3N7O2S/c16-7-5-29-14(12(7)13-20-6-21-23-13)22-9(27)4-25-10-3-8(15(17,18)19)24-26(10)2-1-11(25)28/h1-3,5-6H,4H2,(H,22,27)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352624

(CHEMBL1822305 | US9796706, Compound 139)Show SMILES Cn1cnc(n1)-c1c(Br)csc1NC(=O)CN1C(=O)CCc2ncccc12 Show InChI InChI=1S/C17H15BrN6O2S/c1-23-9-20-16(22-23)15-10(18)8-27-17(15)21-13(25)7-24-12-3-2-6-19-11(12)4-5-14(24)26/h2-3,6,8-9H,4-5,7H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258309

(US9493485, 9-150)Show SMILES CC1(CC2(CCOCC2)SC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |c:12| Show InChI InChI=1S/C21H22ClFN4O2S/c1-20(12-21(30-19(24)27-20)6-8-29-9-7-21)15-10-14(3-4-16(15)23)26-18(28)17-5-2-13(22)11-25-17/h2-5,10-11H,6-9,12H2,1H3,(H2,24,27)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds are also assessed for BACE1 and Cathepsin D activity using an FP Assay. Compounds to be assessed (e.g. compounds as described in the above ... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060233
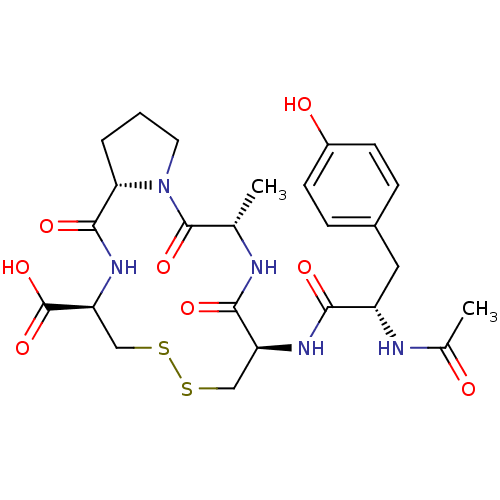
((5S,8R,13R,15aS)-8-[(S)-2-Acetylamino-3-(4-hydroxy...)Show SMILES C[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]2CCCN2C1=O)C(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O Show InChI InChI=1S/C25H33N5O8S2/c1-13-24(36)30-9-3-4-20(30)23(35)29-19(25(37)38)12-40-39-11-18(22(34)26-13)28-21(33)17(27-14(2)31)10-15-5-7-16(32)8-6-15/h5-8,13,17-20,32H,3-4,9-12H2,1-2H3,(H,26,34)(H,27,31)(H,28,33)(H,29,35)(H,37,38)/t13-,17-,18-,19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060230

((5S,8R,13R,15aS)-8-{[(2R,3R)-1-Acetyl-3-(4-hydroxy...)Show SMILES CC(=O)N1CC[C@@H]([C@@H]1C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CO)NC1=O)C(O)=O)c1ccc(O)cc1 Show InChI InChI=1S/C27H35N5O9S2/c1-14(34)31-10-8-17(15-4-6-16(35)7-5-15)22(31)25(38)29-19-12-42-43-13-20(27(40)41)30-24(37)21-3-2-9-32(21)26(39)18(11-33)28-23(19)36/h4-7,17-22,33,35H,2-3,8-13H2,1H3,(H,28,36)(H,29,38)(H,30,37)(H,40,41)/t17-,18+,19+,20+,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352609
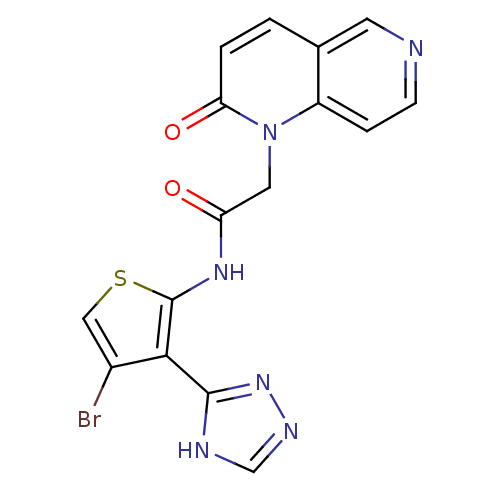
(CHEMBL1822140)Show SMILES Brc1csc(NC(=O)Cn2c3ccncc3ccc2=O)c1-c1nnc[nH]1 Show InChI InChI=1S/C16H11BrN6O2S/c17-10-7-26-16(14(10)15-19-8-20-22-15)21-12(24)6-23-11-3-4-18-5-9(11)1-2-13(23)25/h1-5,7-8H,6H2,(H,21,24)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352626
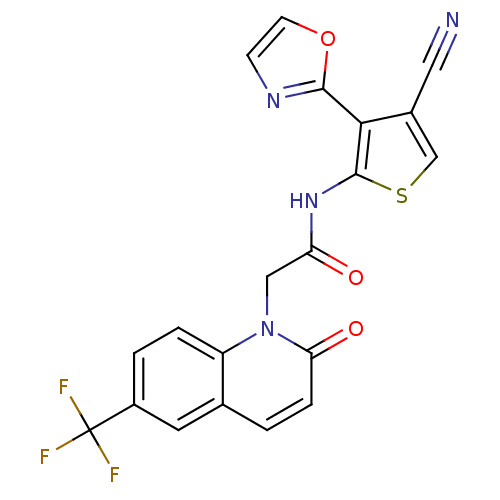
(CHEMBL1822307 | US9796706, Compound 131)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(C#N)c3-c3ncco3)c(=O)ccc2c1 Show InChI InChI=1S/C20H11F3N4O3S/c21-20(22,23)13-2-3-14-11(7-13)1-4-16(29)27(14)9-15(28)26-19-17(12(8-24)10-31-19)18-25-5-6-30-18/h1-7,10H,9H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258287
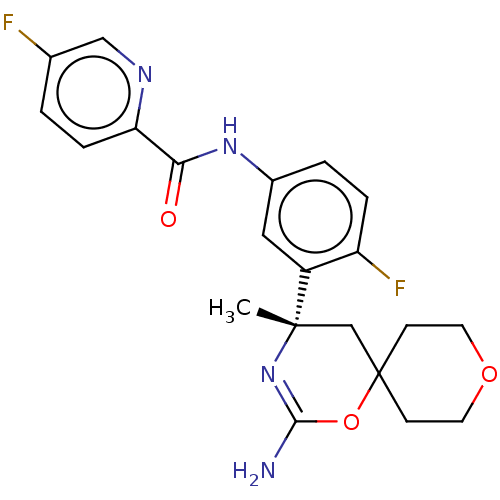
(US9493485, 9-128)Show SMILES C[C@]1(CC2(CCOCC2)OC(N)=N1)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,c:12| Show InChI InChI=1S/C21H22F2N4O3/c1-20(12-21(30-19(24)27-20)6-8-29-9-7-21)15-10-14(3-4-16(15)23)26-18(28)17-5-2-13(22)11-25-17/h2-5,10-11H,6-9,12H2,1H3,(H2,24,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
BACE1 activity can be assessed in an AlphaScreenŽ assay. Compounds to be assessed (e.g. compounds as described in the above examples) are serially di... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16016

(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p38alpha after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352610
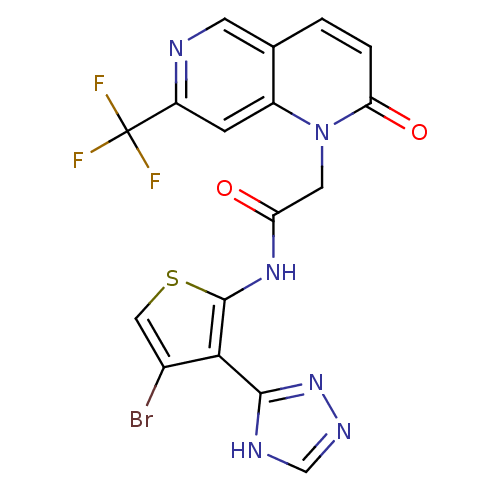
(CHEMBL1822141)Show SMILES FC(F)(F)c1cc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2cn1 Show InChI InChI=1S/C17H10BrF3N6O2S/c18-9-6-30-16(14(9)15-23-7-24-26-15)25-12(28)5-27-10-3-11(17(19,20)21)22-4-8(10)1-2-13(27)29/h1-4,6-7H,5H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50338292
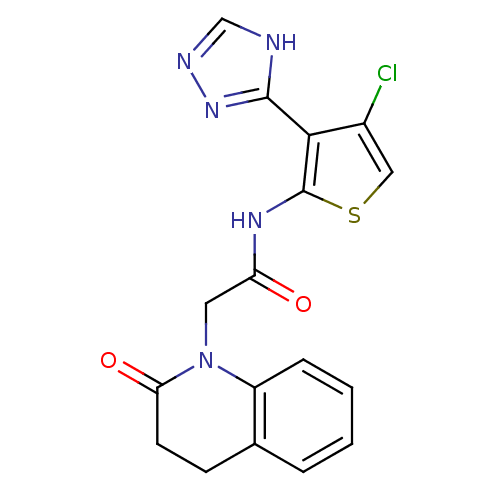
(CHEMBL1682014 | N-(4-chloro-3-(1H-1,2,4-triazol-5-...)Show SMILES Clc1csc(NC(=O)CN2C(=O)CCc3ccccc23)c1-c1nnc[nH]1 Show InChI InChI=1S/C17H14ClN5O2S/c18-11-8-26-17(15(11)16-19-9-20-22-16)21-13(24)7-23-12-4-2-1-3-10(12)5-6-14(23)25/h1-4,8-9H,5-7H2,(H,21,24)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 1838-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.046
BindingDB Entry DOI: 10.7270/Q2Q52PX0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060168
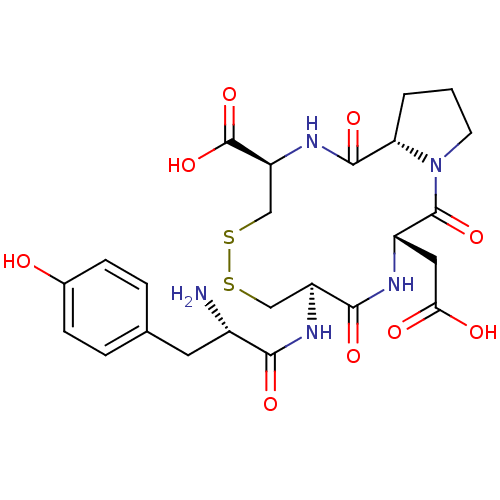
((5S,8R,13R,15aS)-8-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC1=O)C(O)=O Show InChI InChI=1S/C24H31N5O9S2/c25-14(8-12-3-5-13(30)6-4-12)20(33)27-16-10-39-40-11-17(24(37)38)28-22(35)18-2-1-7-29(18)23(36)15(9-19(31)32)26-21(16)34/h3-6,14-18,30H,1-2,7-11,25H2,(H,26,34)(H,27,33)(H,28,35)(H,31,32)(H,37,38)/t14-,15-,16-,17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50060179
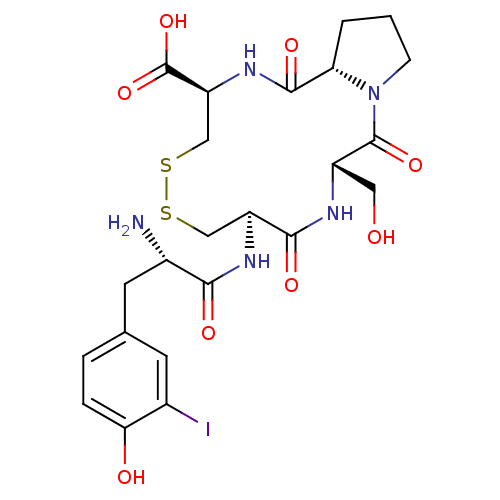
((5S,8R,13R,15aS)-8-[(S)-2-Amino-3-(4-hydroxy-3-iod...)Show SMILES N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CO)NC1=O)C(O)=O Show InChI InChI=1S/C23H30IN5O8S2/c24-12-6-11(3-4-18(12)31)7-13(25)19(32)27-15-9-38-39-10-16(23(36)37)28-21(34)17-2-1-5-29(17)22(35)14(8-30)26-20(15)33/h3-4,6,13-17,30-31H,1-2,5,7-10,25H2,(H,26,33)(H,27,32)(H,28,34)(H,36,37)/t13-,14-,15-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified alpha-4 beta-1 binding to VCAM-1 was determined in an ELISA assay. |
J Med Chem 40: 3359-68 (1997)
Article DOI: 10.1021/jm970175s
BindingDB Entry DOI: 10.7270/Q298889J |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258304

(US9493485, 9-145)Show SMILES C[C@]1(CC2(CCC2)SC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:10| Show InChI InChI=1S/C20H20ClFN4OS/c1-19(11-20(7-2-8-20)28-18(23)26-19)14-9-13(4-5-15(14)22)25-17(27)16-6-3-12(21)10-24-16/h3-6,9-10H,2,7-8,11H2,1H3,(H2,23,26)(H,25,27)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
BACE1 activity can be assessed in an AlphaScreenŽ assay. Compounds to be assessed (e.g. compounds as described in the above examples) are serially di... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM258283

(US9493485, 9-124)Show SMILES C[C@]1(CC2(CCC(F)(F)CC2)OC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:14| Show InChI InChI=1S/C22H22ClF3N4O2/c1-20(12-21(32-19(27)30-20)6-8-22(25,26)9-7-21)15-10-14(3-4-16(15)24)29-18(31)17-5-2-13(23)11-28-17/h2-5,10-11H,6-9,12H2,1H3,(H2,27,30)(H,29,31)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
BACE1 activity can be assessed in an AlphaScreenŽ assay. Compounds to be assessed (e.g. compounds as described in the above examples) are serially di... |
US Patent US9493485 (2016)
BindingDB Entry DOI: 10.7270/Q25B01DD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data