 Found 513 hits with Last Name = 'atadja' and Initial = 'p'
Found 513 hits with Last Name = 'atadja' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350831
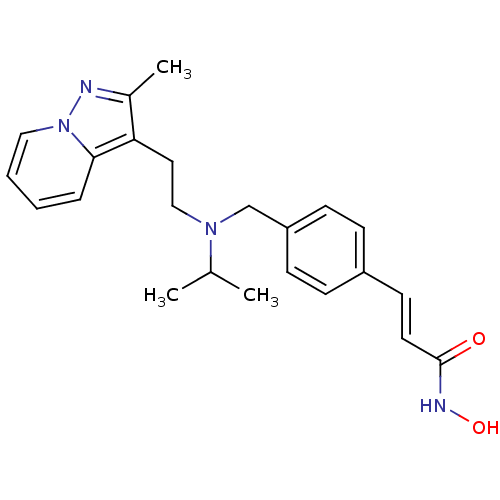
(CHEMBL1819273)Show SMILES CC(C)N(CCc1c(C)nn2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H28N4O2/c1-17(2)26(15-13-21-18(3)24-27-14-5-4-6-22(21)27)16-20-9-7-19(8-10-20)11-12-23(28)25-29/h4-12,14,17,29H,13,15-16H2,1-3H3,(H,25,28)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350832
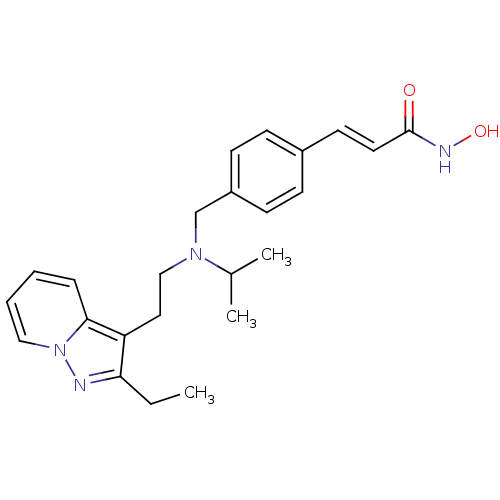
(CHEMBL1819274)Show SMILES CCc1nn2ccccc2c1CCN(Cc1ccc(\C=C\C(=O)NO)cc1)C(C)C Show InChI InChI=1S/C24H30N4O2/c1-4-22-21(23-7-5-6-15-28(23)25-22)14-16-27(18(2)3)17-20-10-8-19(9-11-20)12-13-24(29)26-30/h5-13,15,18,30H,4,14,16-17H2,1-3H3,(H,26,29)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350818
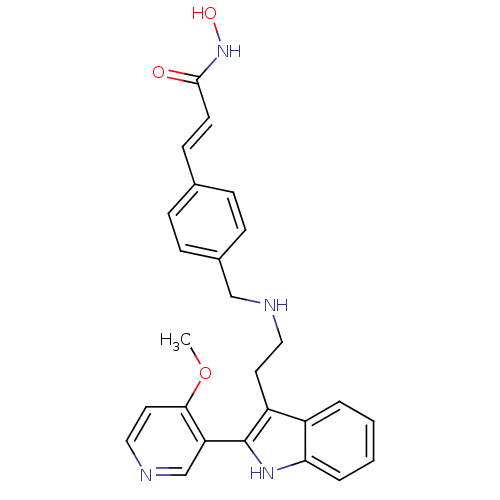
(CHEMBL1819257)Show SMILES COc1ccncc1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H26N4O3/c1-33-24-13-15-28-17-22(24)26-21(20-4-2-3-5-23(20)29-26)12-14-27-16-19-8-6-18(7-9-19)10-11-25(31)30-32/h2-11,13,15,17,27,29,32H,12,14,16H2,1H3,(H,30,31)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350827
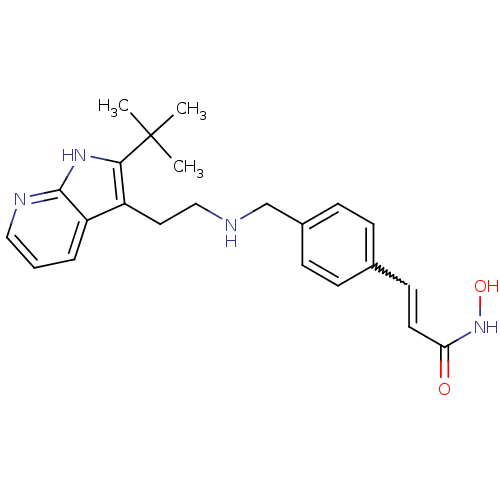
(CHEMBL1819267)Show SMILES CC(C)(C)c1[nH]c2ncccc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:21.22| Show InChI InChI=1S/C23H28N4O2/c1-23(2,3)21-18(19-5-4-13-25-22(19)26-21)12-14-24-15-17-8-6-16(7-9-17)10-11-20(28)27-29/h4-11,13,24,29H,12,14-15H2,1-3H3,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350835
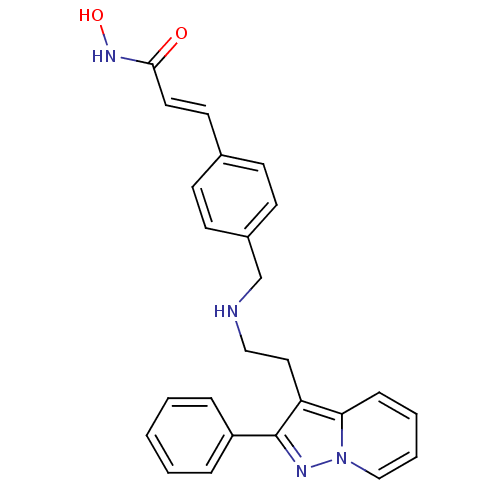
(CHEMBL1819272)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(28-31)14-13-19-9-11-20(12-10-19)18-26-16-15-22-23-8-4-5-17-29(23)27-25(22)21-6-2-1-3-7-21/h1-14,17,26,31H,15-16,18H2,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM172038
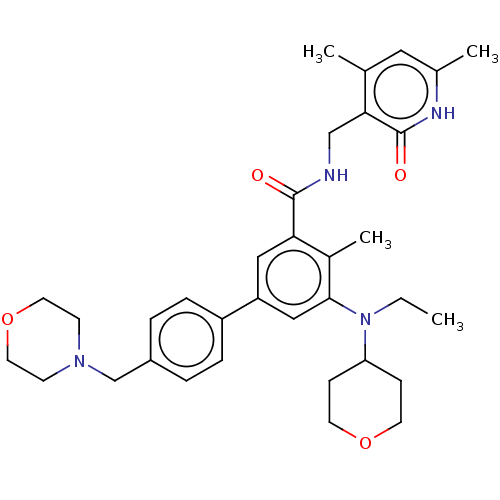
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonist activity was determined against hPR (human progesterone receptor) compared to that of progesterone (100%) |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350820
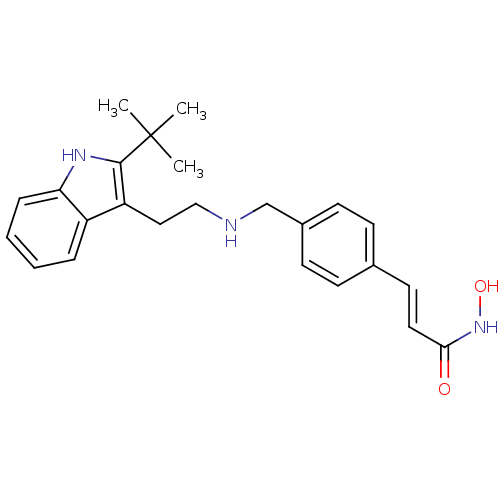
(CHEMBL1819260)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H29N3O2/c1-24(2,3)23-20(19-6-4-5-7-21(19)26-23)14-15-25-16-18-10-8-17(9-11-18)12-13-22(28)27-29/h4-13,25-26,29H,14-16H2,1-3H3,(H,27,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314628
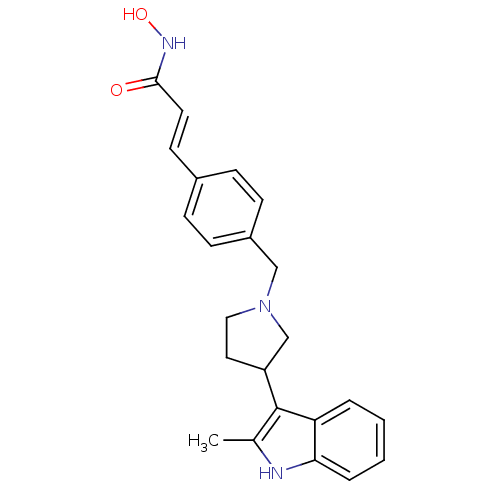
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C23H25N3O2/c1-16-23(20-4-2-3-5-21(20)24-16)19-12-13-26(15-19)14-18-8-6-17(7-9-18)10-11-22(27)25-28/h2-11,19,24,28H,12-15H2,1H3,(H,25,27)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350830
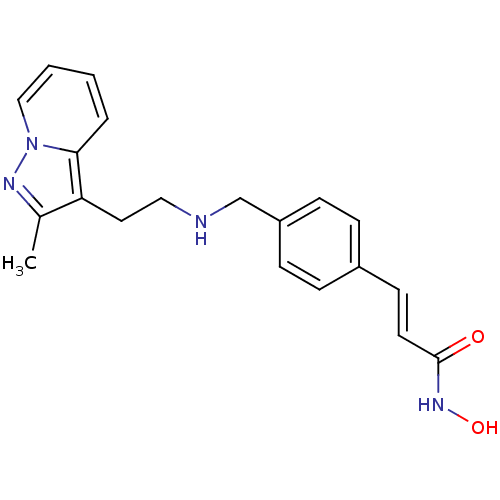
(CHEMBL1819270)Show InChI InChI=1S/C20H22N4O2/c1-15-18(19-4-2-3-13-24(19)22-15)11-12-21-14-17-7-5-16(6-8-17)9-10-20(25)23-26/h2-10,13,21,26H,11-12,14H2,1H3,(H,23,25)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350833
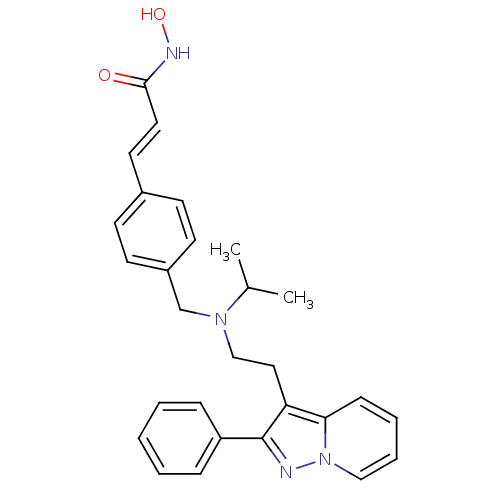
(CHEMBL1819275)Show SMILES CC(C)N(CCc1c(nn2ccccc12)-c1ccccc1)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C28H30N4O2/c1-21(2)31(20-23-13-11-22(12-14-23)15-16-27(33)30-34)19-17-25-26-10-6-7-18-32(26)29-28(25)24-8-4-3-5-9-24/h3-16,18,21,34H,17,19-20H2,1-2H3,(H,30,33)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130
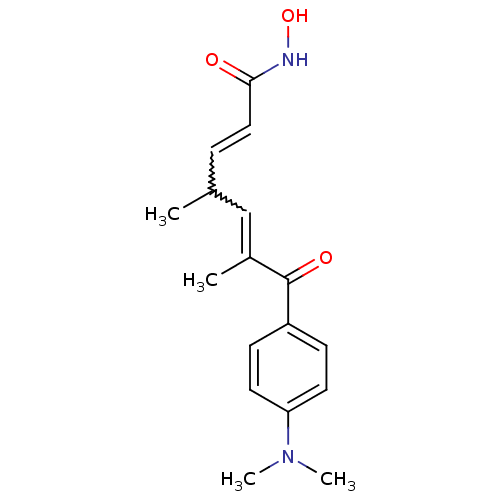
((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 using rhodamine as substrate after 1 hrs by fluorescence assay |
Bioorg Med Chem 19: 4626-34 (2011)
Article DOI: 10.1016/j.bmc.2011.06.030
BindingDB Entry DOI: 10.7270/Q2319W8N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314637

((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C27H33N3O2/c1-27(2,3)26-25(22-8-4-5-9-23(22)28-26)21-7-6-16-30(18-21)17-20-12-10-19(11-13-20)14-15-24(31)29-32/h4-5,8-15,21,28,32H,6-7,16-18H2,1-3H3,(H,29,31)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314630

((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCCC1c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H27N3O2/c1-17-20(21-5-2-3-6-22(21)25-17)14-16-27-15-4-7-23(27)19-11-8-18(9-12-19)10-13-24(28)26-29/h2-3,5-6,8-13,23,25,29H,4,7,14-16H2,1H3,(H,26,28)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364
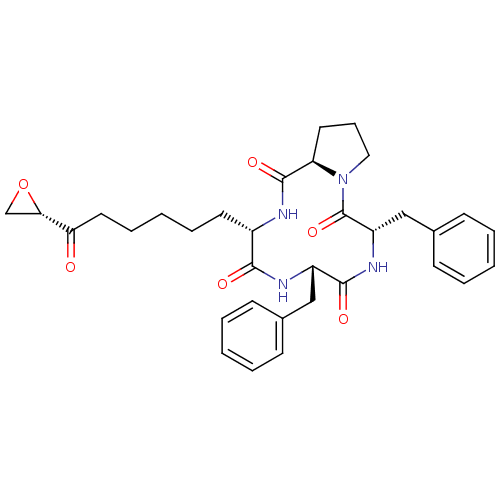
(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the partially purified HDAC enzyme by 50% obtained from H1299 cell lysate |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350821
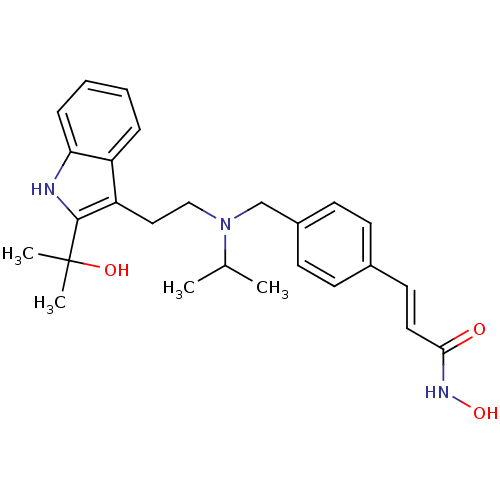
(CHEMBL1819261)Show SMILES CC(C)N(CCc1c([nH]c2ccccc12)C(C)(C)O)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H33N3O3/c1-18(2)29(17-20-11-9-19(10-12-20)13-14-24(30)28-32)16-15-22-21-7-5-6-8-23(21)27-25(22)26(3,4)31/h5-14,18,27,31-32H,15-17H2,1-4H3,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 using rhodamine as substrate after 1 hrs by fluorescence assay |
Bioorg Med Chem 19: 4626-34 (2011)
Article DOI: 10.1016/j.bmc.2011.06.030
BindingDB Entry DOI: 10.7270/Q2319W8N |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314627
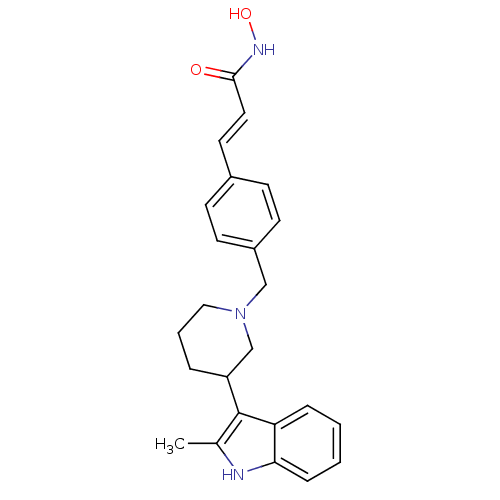
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...)Show SMILES Cc1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C24H27N3O2/c1-17-24(21-6-2-3-7-22(21)25-17)20-5-4-14-27(16-20)15-19-10-8-18(9-11-19)12-13-23(28)26-29/h2-3,6-13,20,25,29H,4-5,14-16H2,1H3,(H,26,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350826
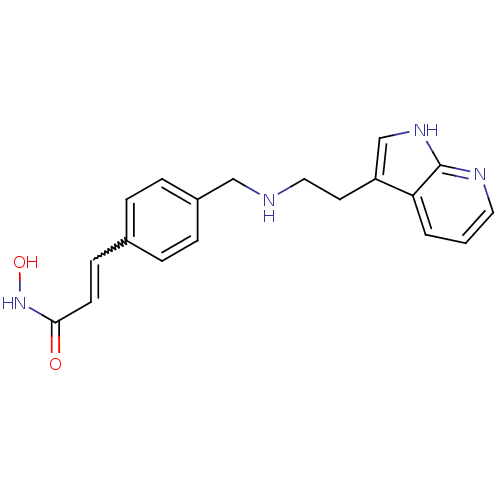
(CHEMBL1819266)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3ncccc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-19-17(16)2-1-10-21-19/h1-8,10,13,20,25H,9,11-12H2,(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human Histone deacetylase (HDAC) enzyme by 50% |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350838
(CHEMBL1819271)Show InChI InChI=1S/C21H24N4O2/c1-2-19-18(20-5-3-4-14-25(20)23-19)12-13-22-15-17-8-6-16(7-9-17)10-11-21(26)24-27/h3-11,14,22,27H,2,12-13,15H2,1H3,(H,24,26)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
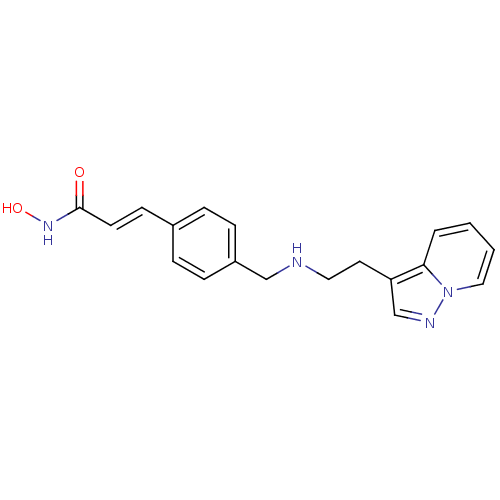
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350829
(CHEMBL1819269)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-23-12-2-1-3-18(17)23/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236010

(CHEMBL4103319)Show SMILES C(Nc1ncc(-c2ccc(CN3CCCC3)cc2)c2nncn12)c1ccco1 Show InChI InChI=1S/C21H22N6O/c1-2-10-26(9-1)14-16-5-7-17(8-6-16)19-13-23-21(27-15-24-25-20(19)27)22-12-18-4-3-11-28-18/h3-8,11,13,15H,1-2,9-10,12,14H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
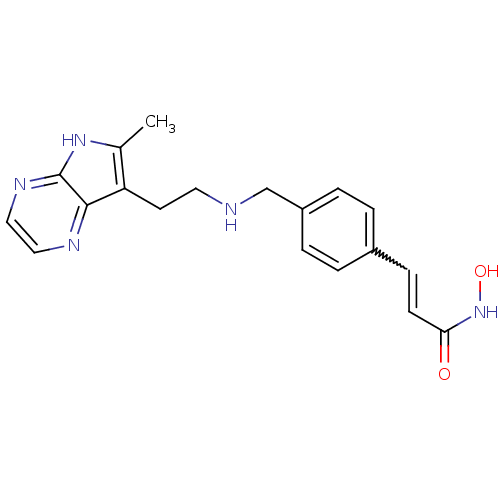
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350824
(CHEMBL1819264)Show SMILES Cc1[nH]c2nccnc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:18.19| Show InChI InChI=1S/C19H21N5O2/c1-13-16(18-19(23-13)22-11-10-21-18)8-9-20-12-15-4-2-14(3-5-15)6-7-17(25)24-26/h2-7,10-11,20,26H,8-9,12H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235990

(CHEMBL4088683)Show SMILES CN(C)Cc1ccc(cc1)-c1cc(C#N)c(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C21H20N6O/c1-26(2)13-15-5-7-16(8-6-15)19-10-17(11-22)20(27-14-24-25-21(19)27)23-12-18-4-3-9-28-18/h3-10,14,23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
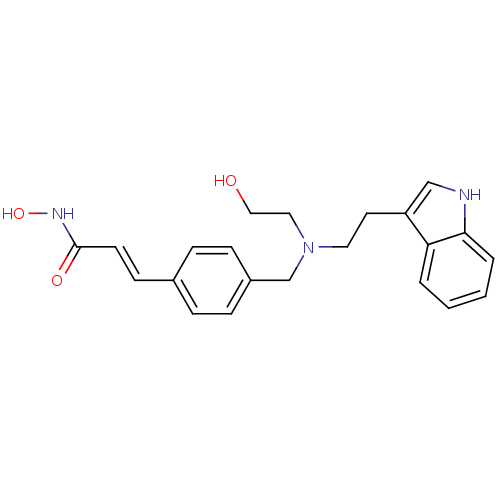
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428
((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
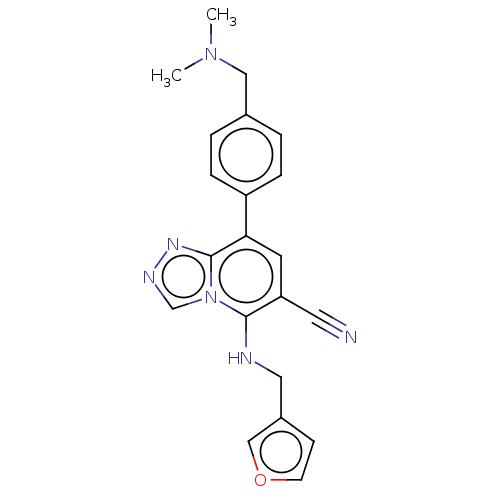
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235996
(CHEMBL4079806)Show SMILES CN(C)Cc1ccc(cc1)-c1cc(C#N)c(NCc2ccoc2)n2cnnc12 Show InChI InChI=1S/C21H20N6O/c1-26(2)12-15-3-5-17(6-4-15)19-9-18(10-22)20(27-14-24-25-21(19)27)23-11-16-7-8-28-13-16/h3-9,13-14,23H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISA |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474360
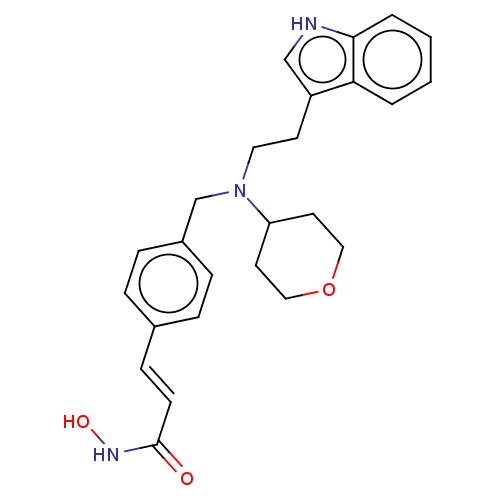
(CHEMBL357231)Show SMILES ONC(=O)\C=C\c1ccc(CN(CCc2c[nH]c3ccccc23)C2CCOCC2)cc1 Show InChI InChI=1S/C25H29N3O3/c29-25(27-30)10-9-19-5-7-20(8-6-19)18-28(22-12-15-31-16-13-22)14-11-21-17-26-24-4-2-1-3-23(21)24/h1-10,17,22,26,30H,11-16,18H2,(H,27,29)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314640
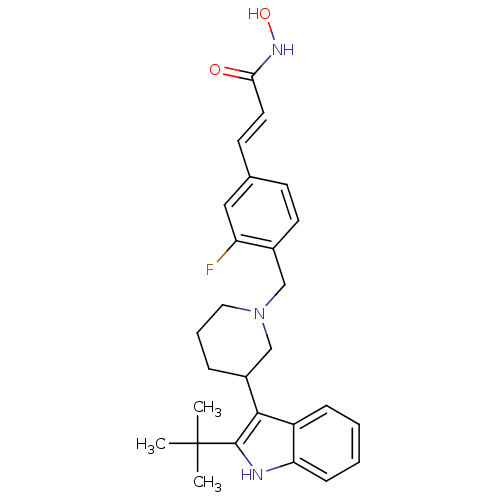
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 3
(Homo sapiens) | BDBM50247381
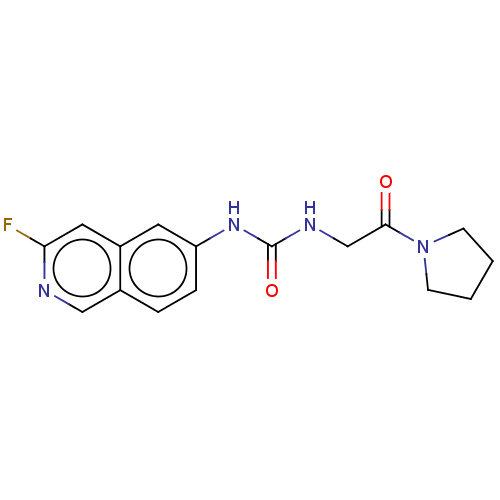
(CHEMBL4079503)Show InChI InChI=1S/C16H17FN4O2/c17-14-8-12-7-13(4-3-11(12)9-18-14)20-16(23)19-10-15(22)21-5-1-2-6-21/h3-4,7-9H,1-2,5-6,10H2,(H2,19,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 (unknown origin) using C-terminally biotinylated histone H4 as substrate in presence of [3H]S-adenosylmethionine by scintillation... |
J Med Chem 61: 1204-1217 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01674
BindingDB Entry DOI: 10.7270/Q24J0HK6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350828
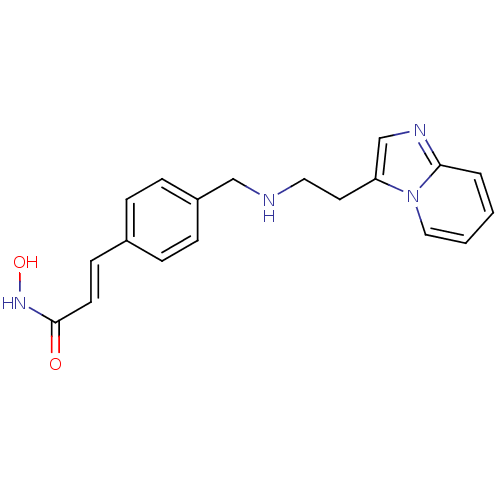
(CHEMBL1819268)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-18-3-1-2-12-23(17)18/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation counting |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350822
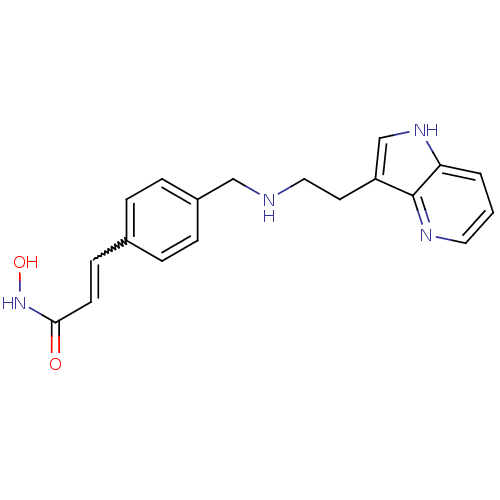
(CHEMBL1819262)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3cccnc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-17-2-1-10-21-19(16)17/h1-8,10,13,20,22,25H,9,11-12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314636
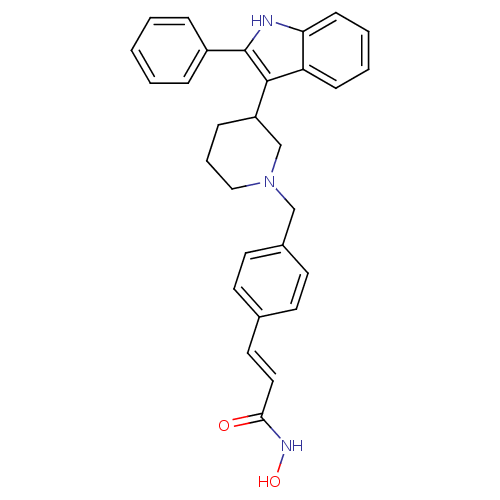
((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N3O2/c33-27(31-34)17-16-21-12-14-22(15-13-21)19-32-18-6-9-24(20-32)28-25-10-4-5-11-26(25)30-29(28)23-7-2-1-3-8-23/h1-5,7-8,10-17,24,30,34H,6,9,18-20H2,(H,31,33)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314635

((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C23H25N3O2/c27-23(25-28)12-11-17-7-9-18(10-8-17)15-26-13-3-4-19(16-26)21-14-24-22-6-2-1-5-20(21)22/h1-2,5-12,14,19,24,28H,3-4,13,15-16H2,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350813
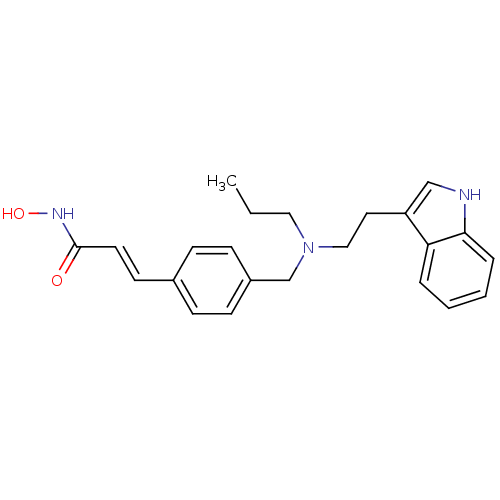
(CHEMBL1819141)Show SMILES CCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O2/c1-2-14-26(15-13-20-16-24-22-6-4-3-5-21(20)22)17-19-9-7-18(8-10-19)11-12-23(27)25-28/h3-12,16,24,28H,2,13-15,17H2,1H3,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350817
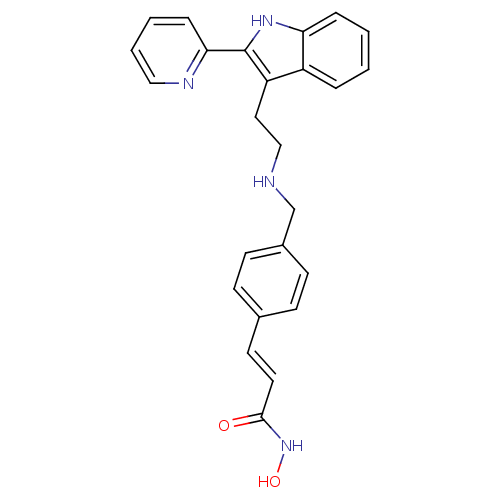
(CHEMBL1819256)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccn2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(29-31)13-12-18-8-10-19(11-9-18)17-26-16-14-21-20-5-1-2-6-22(20)28-25(21)23-7-3-4-15-27-23/h1-13,15,26,28,31H,14,16-17H2,(H,29,30)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350816
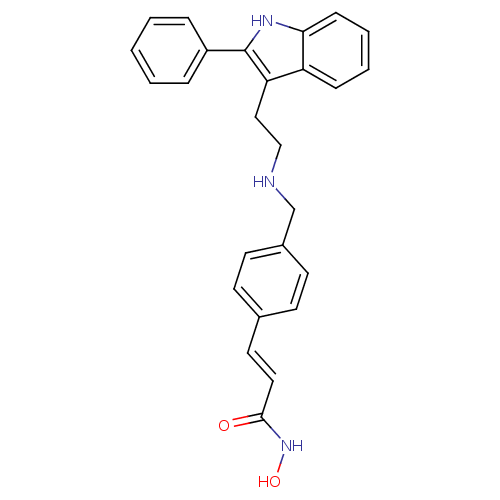
(CHEMBL1819255)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C26H25N3O2/c30-25(29-31)15-14-19-10-12-20(13-11-19)18-27-17-16-23-22-8-4-5-9-24(22)28-26(23)21-6-2-1-3-7-21/h1-15,27-28,31H,16-18H2,(H,29,30)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50236013

(CHEMBL4081748)Show InChI InChI=1S/C19H20N6O/c1-24(2)12-14-5-7-15(8-6-14)17-11-21-19(25-13-22-23-18(17)25)20-10-16-4-3-9-26-16/h3-9,11,13H,10,12H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged EED (76 to 441 residues) (unknown origin) expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL asses... |
J Med Chem 60: 2215-2226 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01576
BindingDB Entry DOI: 10.7270/Q20G3NDT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350810
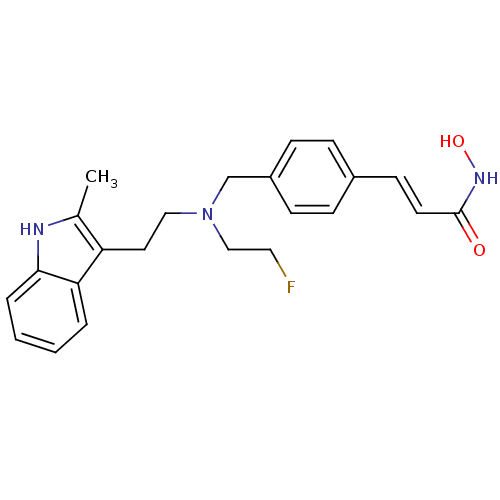
(CHEMBL1819138)Show SMILES Cc1[nH]c2ccccc2c1CCN(CCF)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H26FN3O2/c1-17-20(21-4-2-3-5-22(21)25-17)12-14-27(15-13-24)16-19-8-6-18(7-9-19)10-11-23(28)26-29/h2-11,25,29H,12-16H2,1H3,(H,26,28)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314633

(CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-8-6-17(14-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged HDAC2 (unknown origin) expressed in SF21 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation ... |
Drug Metab Dispos 40: 1041-50 (2012)
Article DOI: 10.1124/dmd.111.043620
BindingDB Entry DOI: 10.7270/Q2SF2XX8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474337

(CHEMBL141502)Show SMILES COc1ccc2[nH]cc(CCNCc3ccc(\C=C\C(=O)NO)cc3)c2c1 Show InChI InChI=1S/C21H23N3O3/c1-27-18-7-8-20-19(12-18)17(14-23-20)10-11-22-13-16-4-2-15(3-5-16)6-9-21(25)24-26/h2-9,12,14,22-23,26H,10-11,13H2,1H3,(H,24,25)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474333

(CHEMBL140013)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c[nH]c3ccc(F)cc23)cc1 Show InChI InChI=1S/C20H20FN3O2/c21-17-6-7-19-18(11-17)16(13-23-19)9-10-22-12-15-3-1-14(2-4-15)5-8-20(25)24-26/h1-8,11,13,22-23,26H,9-10,12H2,(H,24,25)/b8-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314643
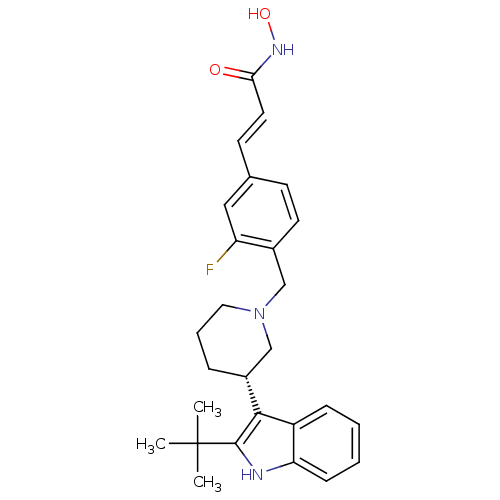
((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data