

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50505576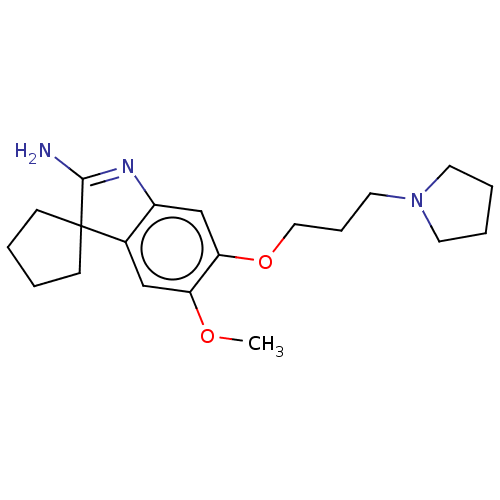 (CHEMBL4458252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) by scintillation proximity Assay | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C [1-765] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D [1-775] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320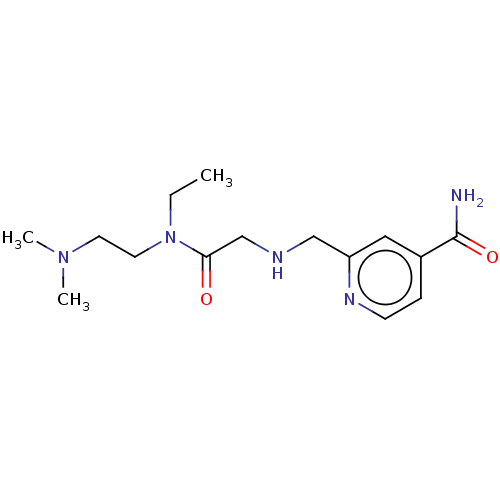 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM195612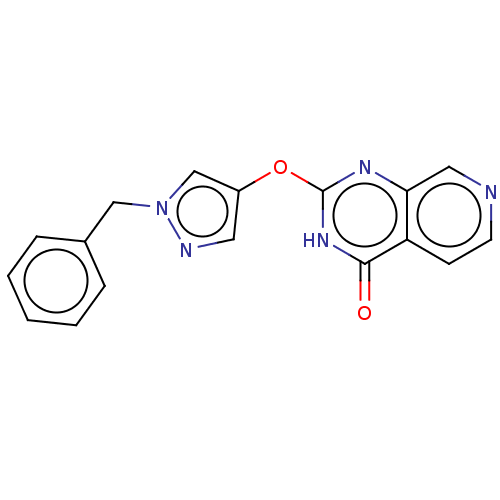 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505574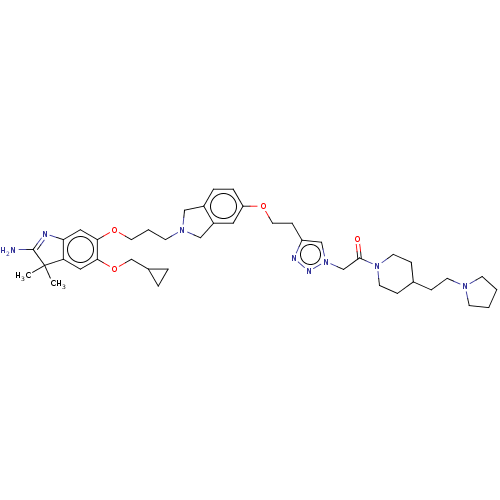 (CHEMBL4451634) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505583 (CHEMBL4573889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191599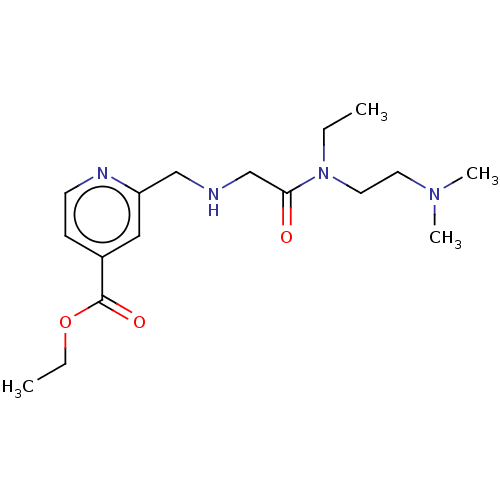 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505578 (CHEMBL4570122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C [1-765] (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5D [1-775] (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50505582 (CHEMBL4465568) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) by scintillation proximity Assay | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505573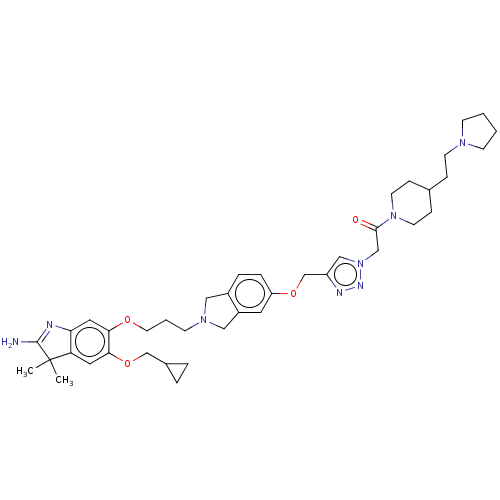 (CHEMBL4577068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 6B (Homo sapiens (Human)) | BDBM60875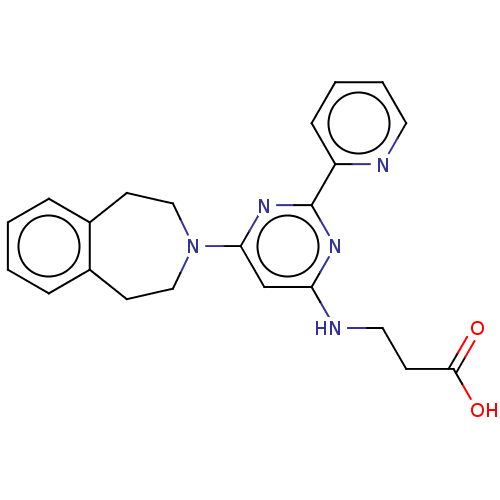 (3-((6-(4,5-Dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50446376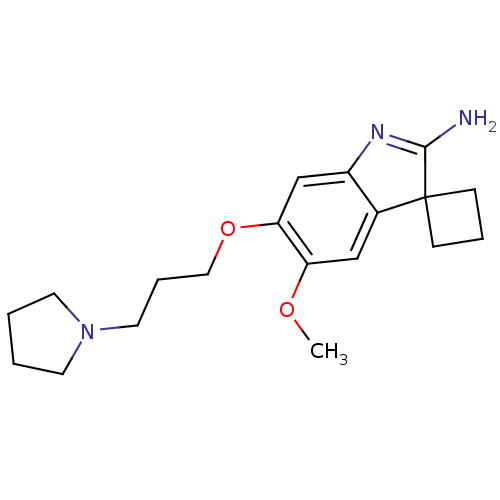 (CHEMBL3109630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Binding affinity to recombinant human His-tagged SPIN1 (49 to 262 residues) expressed in Escherichia coli BL21 (DE3) incubated for 30 mins by AlphaLI... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM191599 (KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223319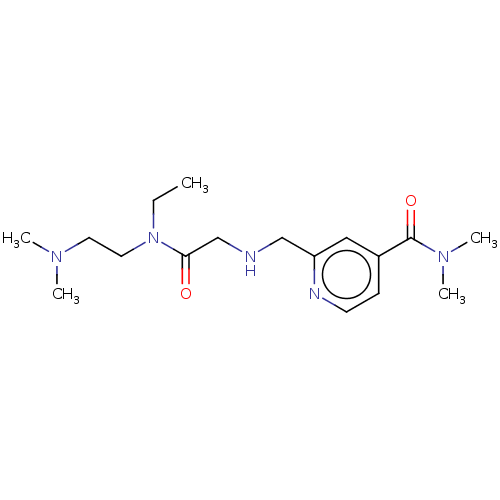 (KDOAM-29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 3A (Homo sapiens (Human)) | BDBM26106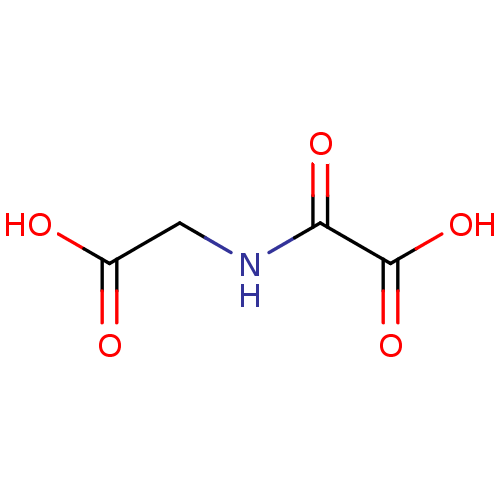 (CHEMBL90852 | N-oxalyl glycine, 1a | NOG | Oxalylg...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223319 (KDOAM-29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM223318 (KDOAM-28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223319 (KDOAM-29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505579 (CHEMBL4591023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505571 (CHEMBL4528213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spindlin-1 (Homo sapiens) | BDBM50505570 (CHEMBL4476309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of trimethylated biotinylated histone H3 peptide from recombinant human His-tagged SPIN1 (26 to 262 residues) expressed in Escherichia c... | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM60875 (3-((6-(4,5-Dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |