 Found 59 hits with Last Name = 'aubertin' and Initial = 'am'
Found 59 hits with Last Name = 'aubertin' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029662

(3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-ethyl-6-...)Show InChI InChI=1S/C16H20N2OS/c1-5-13-11(4)18-16(19)14(17)15(13)20-12-7-9(2)6-10(3)8-12/h6-8H,5,17H2,1-4H3,(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10908
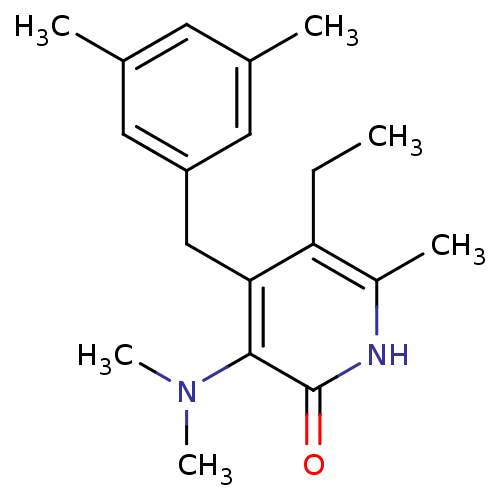
(3-(dimethylamino)-4-[(3,5-dimethylphenyl)methyl]-5...)Show InChI InChI=1S/C19H26N2O/c1-7-16-14(4)20-19(22)18(21(5)6)17(16)11-15-9-12(2)8-13(3)10-15/h8-10H,7,11H2,1-6H3,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10903
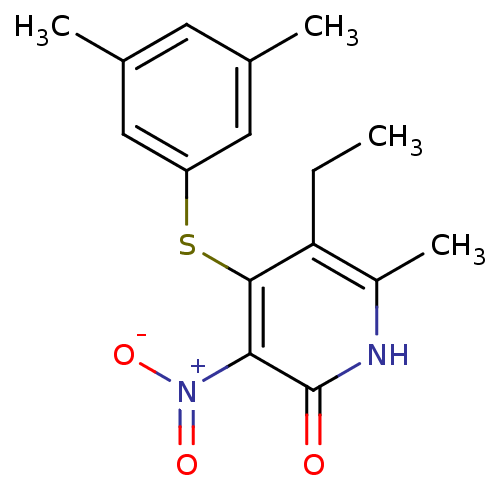
(4-[(3,5-dimethylphenyl)sulfanyl]-5-ethyl-6-methyl-...)Show SMILES CCc1c(C)[nH]c(=O)c(c1Sc1cc(C)cc(C)c1)[N+]([O-])=O Show InChI InChI=1S/C16H18N2O3S/c1-5-13-11(4)17-16(19)14(18(20)21)15(13)22-12-7-9(2)6-10(3)8-12/h6-8H,5H2,1-4H3,(H,17,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029665

(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-3...)Show SMILES CCc1c(C)[n-]c(=[OH+])c(c1Sc1cc(C)cc(C)c1)[N+]([O-])=O Show InChI InChI=1S/C16H18N2O3S/c1-5-13-11(4)17-16(19)14(18(20)21)15(13)22-12-7-9(2)6-10(3)8-12/h6-8H,5H2,1-4H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10906
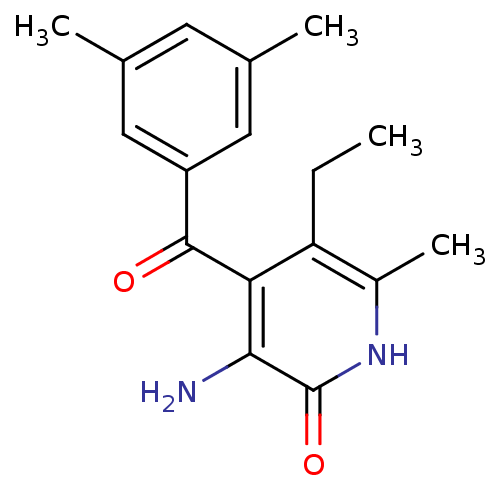
(3-Amino-4-(3,5-dimethylbenzoyl)-5-ethyl-6-methylpy...)Show InChI InChI=1S/C17H20N2O2/c1-5-13-11(4)19-17(21)15(18)14(13)16(20)12-7-9(2)6-10(3)8-12/h6-8H,5,18H2,1-4H3,(H,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10905

(3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...)Show InChI InChI=1S/C17H22N2O/c1-5-14-12(4)19-17(20)16(18)15(14)9-13-7-10(2)6-11(3)8-13/h6-8H,5,9,18H2,1-4H3,(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029660

(3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-methyl-1...)Show InChI InChI=1S/C14H16N2OS/c1-8-4-9(2)6-11(5-8)18-13-10(3)7-16-14(17)12(13)15/h4-7H,15H2,1-3H3,(H,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10907
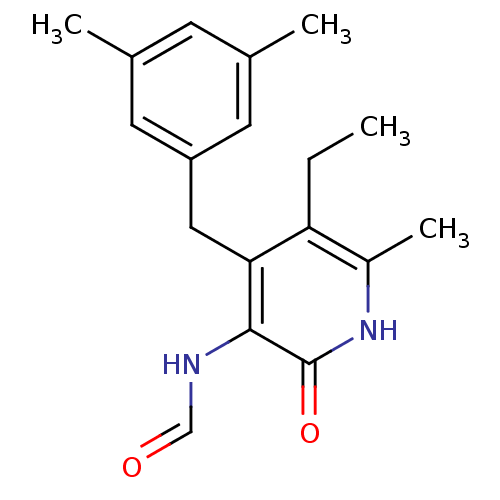
(4-(3,5-Dimethylbenzyl)-5-ethyl-3-formamido-6-methy...)Show InChI InChI=1S/C18H22N2O2/c1-5-15-13(4)20-18(22)17(19-10-21)16(15)9-14-7-11(2)6-12(3)8-14/h6-8,10H,5,9H2,1-4H3,(H,19,21)(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10904
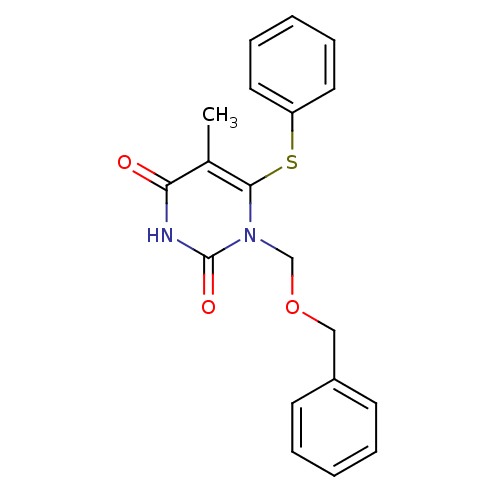
(1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...)Show InChI InChI=1S/C19H18N2O3S/c1-14-17(22)20-19(23)21(13-24-12-15-8-4-2-5-9-15)18(14)25-16-10-6-3-7-11-16/h2-11H,12-13H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029678
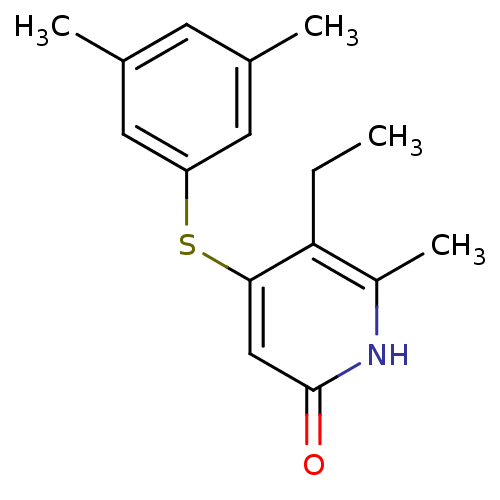
(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-1...)Show InChI InChI=1S/C16H19NOS/c1-5-14-12(4)17-16(18)9-15(14)19-13-7-10(2)6-11(3)8-13/h6-9H,5H2,1-4H3,(H,17,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM10904

(1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...)Show InChI InChI=1S/C19H18N2O3S/c1-14-17(22)20-19(23)21(13-24-12-15-8-4-2-5-9-15)18(14)25-16-10-6-3-7-11-16/h2-11H,12-13H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029663
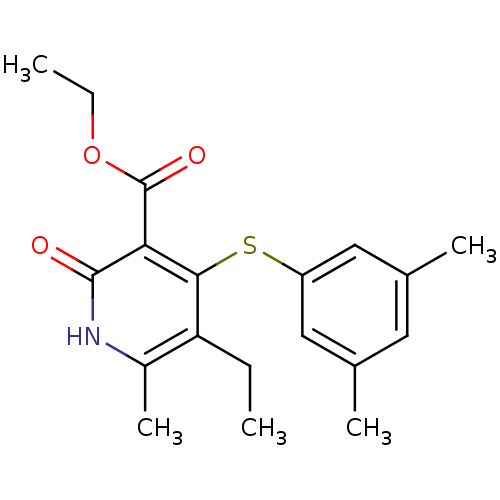
(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-2...)Show SMILES CCOC(=O)c1c(Sc2cc(C)cc(C)c2)c(CC)c(C)[nH]c1=O Show InChI InChI=1S/C19H23NO3S/c1-6-15-13(5)20-18(21)16(19(22)23-7-2)17(15)24-14-9-11(3)8-12(4)10-14/h8-10H,6-7H2,1-5H3,(H,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471649

(CHEMBL3350555)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3CNC(=O)c3c3c4ccccc4[nH]c3c12.CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 |r| Show InChI InChI=1S/2C27H25N3O6/c1-35-25-17(11-31)36-27(24(33)23(25)32)30-16-9-5-3-7-13(16)18-14-10-28-26(34)20(14)19-12-6-2-4-8-15(12)29-21(19)22(18)30;1-35-25-17(11-31)36-27(24(33)23(25)32)30-16-9-5-3-7-13(16)19-20-14(10-28-26(20)34)18-12-6-2-4-8-15(12)29-21(18)22(19)30/h2*2-9,17,23-25,27,29,31-33H,10-11H2,1H3,(H,28,34)/t2*17-,23-,24-,25-,27-/m11/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029672
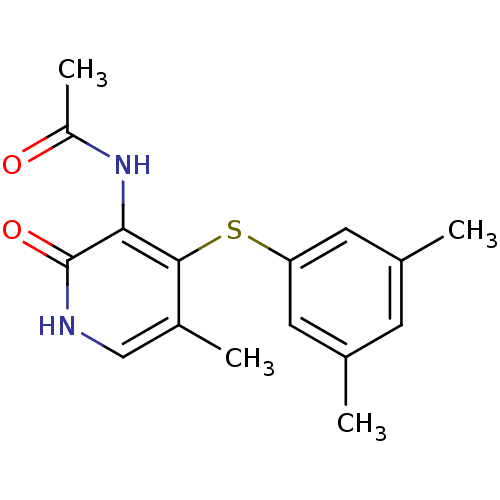
(CHEMBL341880 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...)Show InChI InChI=1S/C16H18N2O2S/c1-9-5-10(2)7-13(6-9)21-15-11(3)8-17-16(20)14(15)18-12(4)19/h5-8H,1-4H3,(H,17,20)(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029679
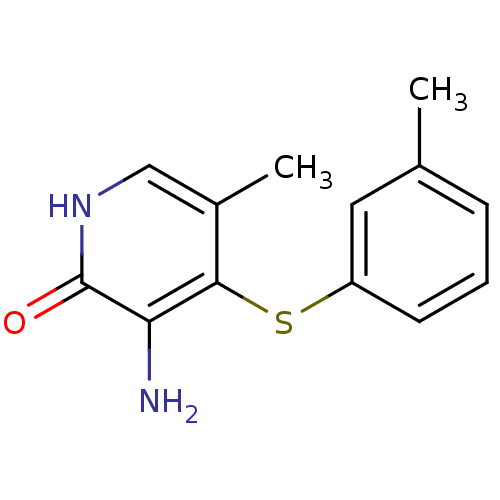
(3-Amino-5-methyl-4-m-tolylsulfanyl-1H-pyridin-2-on...)Show InChI InChI=1S/C13H14N2OS/c1-8-4-3-5-10(6-8)17-12-9(2)7-15-13(16)11(12)14/h3-7H,14H2,1-2H3,(H,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029671
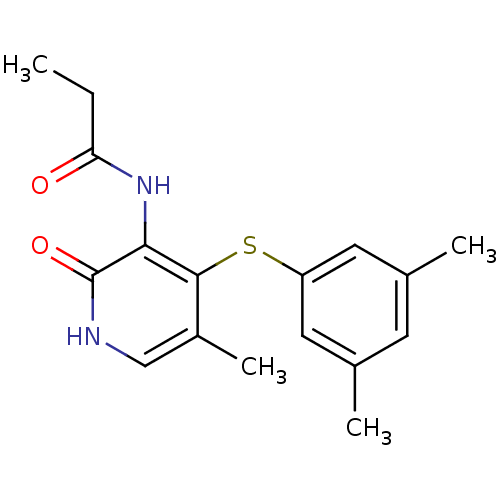
(CHEMBL126931 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...)Show InChI InChI=1S/C17H20N2O2S/c1-5-14(20)19-15-16(12(4)9-18-17(15)21)22-13-7-10(2)6-11(3)8-13/h6-9H,5H2,1-4H3,(H,18,21)(H,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50004152
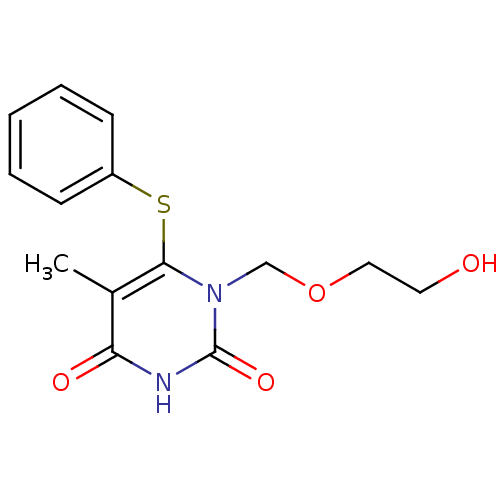
((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029666
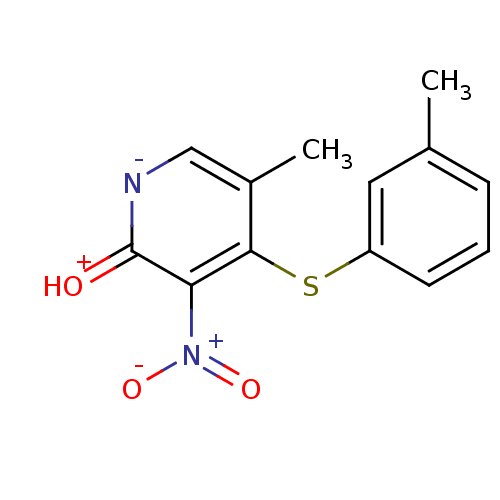
(5-Methyl-3-nitro-4-m-tolylsulfanyl-1H-pyridin-2-on...)Show SMILES Cc1cccc(Sc2c(C)c[n-]c(=[OH+])c2[N+]([O-])=O)c1 Show InChI InChI=1S/C13H12N2O3S/c1-8-4-3-5-10(6-8)19-12-9(2)7-14-13(16)11(12)15(17)18/h3-7H,1-2H3,(H,14,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029675
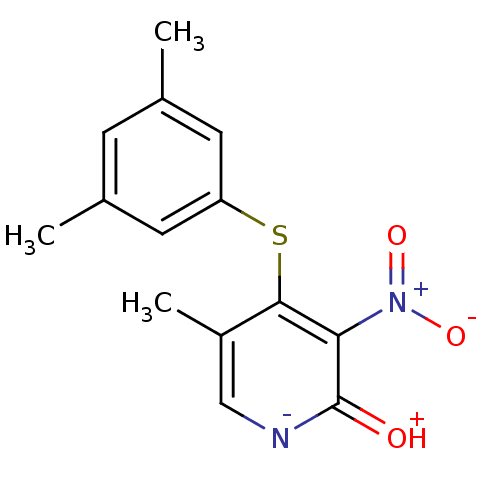
(4-(3,5-Dimethyl-phenylsulfanyl)-5-methyl-3-nitro-1...)Show SMILES Cc1cc(C)cc(Sc2c(C)c[n-]c(=[OH+])c2[N+]([O-])=O)c1 Show InChI InChI=1S/C14H14N2O3S/c1-8-4-9(2)6-11(5-8)20-13-10(3)7-15-14(17)12(13)16(18)19/h4-7H,1-3H3,(H,15,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472524

(CHEMBL345881)Show SMILES COC1C(O)C(OC(=O)CBr)C(OC1CO)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C29H22BrCl2N3O8/c1-41-25-14(9-36)42-29(26(24(25)38)43-15(37)8-30)35-22-11(5-3-7-13(22)32)17-19-18(27(39)34-28(19)40)16-10-4-2-6-12(31)20(10)33-21(16)23(17)35/h2-7,14,24-26,29,33,36,38H,8-9H2,1H3,(H,34,39,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471652
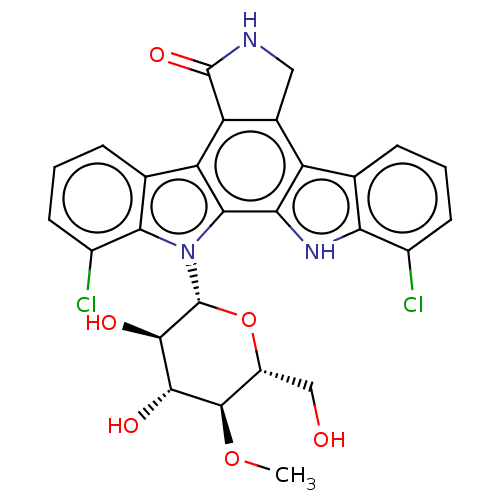
(CHEMBL3350556)Show SMILES CO[C@H]1[C@H](O)[C@@H](O)[C@@H](O[C@@H]1CO)n1c2c(Cl)cccc2c2c3CNC(=O)c3c3c4cccc(Cl)c4[nH]c3c12.CO[C@H]1[C@H](O)[C@@H](O)[C@@H](O[C@@H]1CO)n1c2c(Cl)cccc2c2c3C(=O)NCc3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/2C27H23Cl2N3O6/c1-37-25-15(9-33)38-27(24(35)23(25)34)32-21-11(5-3-7-14(21)29)16-12-8-30-26(36)18(12)17-10-4-2-6-13(28)19(10)31-20(17)22(16)32;1-37-25-15(9-33)38-27(24(35)23(25)34)32-21-11(5-3-7-14(21)29)17-18-12(8-30-26(18)36)16-10-4-2-6-13(28)19(10)31-20(16)22(17)32/h2*2-7,15,23-25,27,31,33-35H,8-9H2,1H3,(H,30,36)/t2*15-,23-,24-,25-,27-/m11/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471651

(CHEMBL3350558)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2c(Cl)cccc2c2c3C(=O)N(C)C(=O)c3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/C28H23Cl2N3O7/c1-32-26(37)17-15-10-5-3-7-12(29)19(10)31-20(15)22-16(18(17)27(32)38)11-6-4-8-13(30)21(11)33(22)28-24(36)23(35)25(39-2)14(9-34)40-28/h3-8,14,23-25,28,31,34-36H,9H2,1-2H3/t14-,23-,24-,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50471650
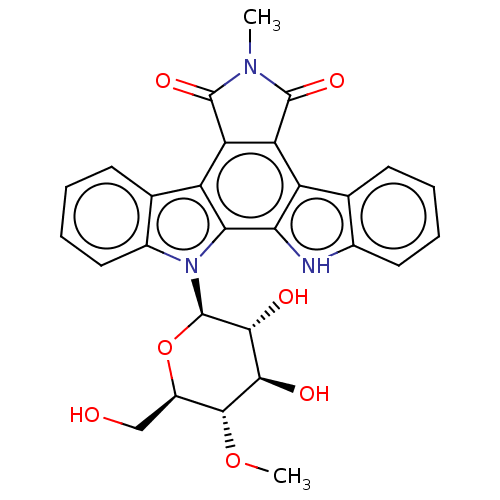
(CHEMBL3350560)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3C(=O)N(C)C(=O)c3c3c4ccccc4[nH]c3c12 |r| Show InChI InChI=1S/C28H25N3O7/c1-30-26(35)19-17-12-7-3-5-9-14(12)29-21(17)22-18(20(19)27(30)36)13-8-4-6-10-15(13)31(22)28-24(34)23(33)25(37-2)16(11-32)38-28/h3-10,16,23-25,28-29,32-34H,11H2,1-2H3/t16-,23-,24-,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 41: 1631-40 (1998)
Article DOI: 10.1021/jm970843+
BindingDB Entry DOI: 10.7270/Q2RV0RF0 |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472527
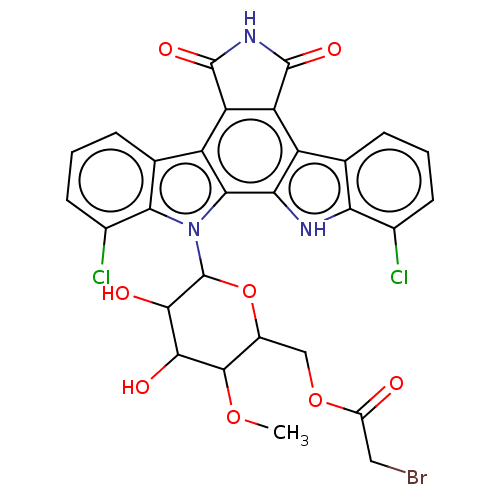
(CHEMBL156776)Show SMILES COC1C(O)C(O)C(OC1COC(=O)CBr)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C29H22BrCl2N3O8/c1-41-26-14(9-42-15(36)8-30)43-29(25(38)24(26)37)35-22-11(5-3-7-13(22)32)17-19-18(27(39)34-28(19)40)16-10-4-2-6-12(31)20(10)33-21(16)23(17)35/h2-7,14,24-26,29,33,37-38H,8-9H2,1H3,(H,34,39,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472525
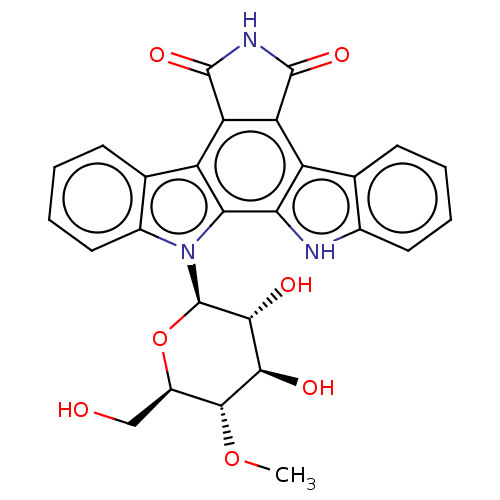
(CHEMBL3350557)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2ccccc2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 |r| Show InChI InChI=1S/C27H23N3O7/c1-36-24-15(10-31)37-27(23(33)22(24)32)30-14-9-5-3-7-12(14)17-19-18(25(34)29-26(19)35)16-11-6-2-4-8-13(11)28-20(16)21(17)30/h2-9,15,22-24,27-28,31-33H,10H2,1H3,(H,29,34,35)/t15-,22-,23-,24-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472530

(CHEMBL154620)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2ccccc2c2c3C(=O)N(CCCCOC(=O)CBr)C(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C33H32BrN3O9/c1-44-30-20(15-38)46-33(29(41)28(30)40)37-19-11-5-3-9-17(19)23-25-24(22-16-8-2-4-10-18(16)35-26(22)27(23)37)31(42)36(32(25)43)12-6-7-13-45-21(39)14-34/h2-5,8-11,20,28-30,33,35,38,40-41H,6-7,12-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472528

(CHEMBL345384)Show SMILES COC1C(CO)OC(C(O)C1O)n1c2c(Cl)cccc2c2c3C(=O)N(CC(=O)CCl)C(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C30H24Cl3N3O8/c1-43-27-16(10-37)44-30(26(40)25(27)39)36-23-13(5-3-7-15(23)33)18-20-19(28(41)35(29(20)42)9-11(38)8-31)17-12-4-2-6-14(32)21(12)34-22(17)24(18)36/h2-7,16,25-27,30,34,37,39-40H,8-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472531
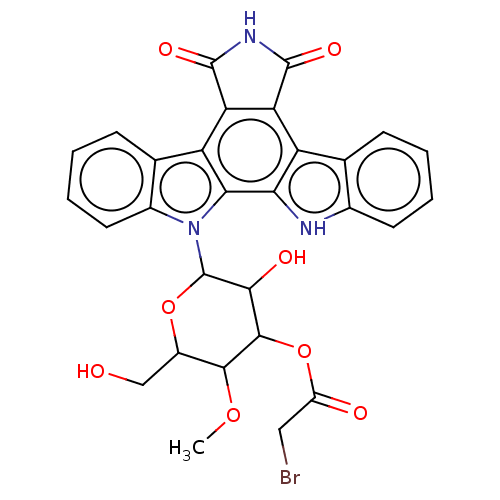
(CHEMBL347195)Show SMILES COC1C(CO)OC(C(O)C1OC(=O)CBr)n1c2ccccc2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C29H24BrN3O8/c1-39-25-16(11-34)40-29(24(36)26(25)41-17(35)10-30)33-15-9-5-3-7-13(15)19-21-20(27(37)32-28(21)38)18-12-6-2-4-8-14(12)31-22(18)23(19)33/h2-9,16,24-26,29,31,34,36H,10-11H2,1H3,(H,32,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472529

(CHEMBL347684)Show SMILES COC1C(CO)OC(C(OC(=O)CBr)C1O)n1c2ccccc2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C29H24BrN3O8/c1-39-25-16(11-34)40-29(26(24(25)36)41-17(35)10-30)33-15-9-5-3-7-13(15)19-21-20(27(37)32-28(21)38)18-12-6-2-4-8-14(12)31-22(18)23(19)33/h2-9,16,24-26,29,31,34,36H,10-11H2,1H3,(H,32,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50472526

(CHEMBL347466)Show SMILES COC1C(CO)OC(C(OC(C)=O)C1O)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 Show InChI InChI=1S/C29H23Cl2N3O8/c1-10(36)41-26-24(37)25(40-2)15(9-35)42-29(26)34-22-12(6-4-8-14(22)31)17-19-18(27(38)33-28(19)39)16-11-5-3-7-13(30)20(11)32-21(16)23(17)34/h3-8,15,24-26,29,32,35,37H,9H2,1-2H3,(H,33,38,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50162287
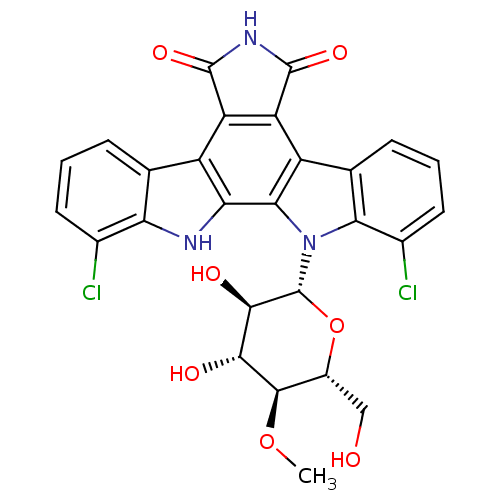
((Rebeccamycin)1,11-dichloro-12-(3,4-dihydroxy-6-hy...)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/C27H21Cl2N3O7/c1-38-24-13(8-33)39-27(23(35)22(24)34)32-20-10(5-3-7-12(20)29)15-17-16(25(36)31-26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)32/h2-7,13,22-24,27,30,33-35H,8H2,1H3,(H,31,36,37)/t13-,22-,23-,24-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Blaise Pascal
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C (PKC) |
J Med Chem 42: 584-92 (1999)
Article DOI: 10.1021/jm980396d
BindingDB Entry DOI: 10.7270/Q2SF2ZXP |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408890
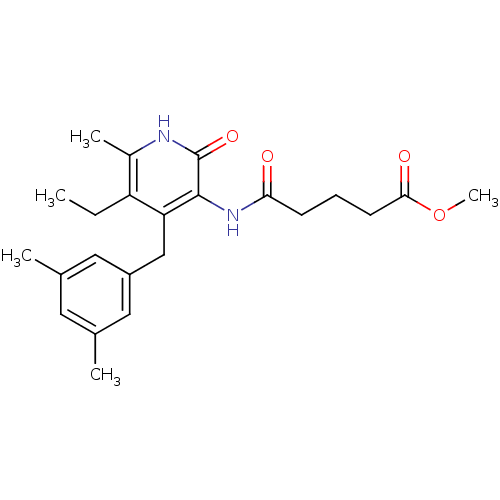
(CHEMBL57301)Show SMILES CCc1c(C)[nH]c(=O)c(NC(=O)CCCC(=O)OC)c1Cc1cc(C)cc(C)c1 Show InChI InChI=1S/C23H30N2O4/c1-6-18-16(4)24-23(28)22(25-20(26)8-7-9-21(27)29-5)19(18)13-17-11-14(2)10-15(3)12-17/h10-12H,6-9,13H2,1-5H3,(H,24,28)(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408891
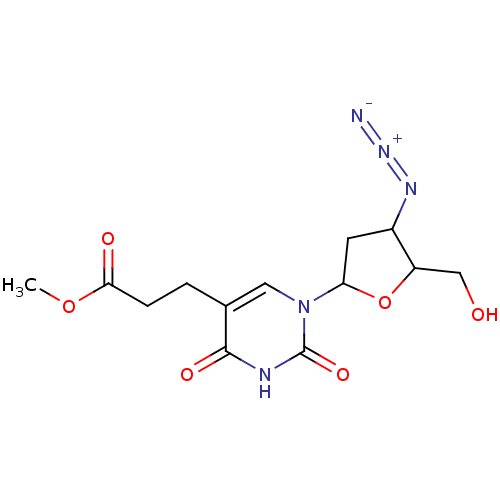
(CHEMBL58891)Show SMILES COC(=O)CCc1cn(C2CC(N=[N+]=[N-])C(CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C13H17N5O6/c1-23-11(20)3-2-7-5-18(13(22)15-12(7)21)10-4-8(16-17-14)9(6-19)24-10/h5,8-10,19H,2-4,6H2,1H3,(H,15,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408892

(CHEMBL418118)Show SMILES CCc1c(C)[nH]c(=O)c(NC(=O)CCCCCCCCCNC(=O)OC(C)(C)C)c1Cc1cc(C)cc(C)c1 Show InChI InChI=1S/C32H49N3O4/c1-8-26-24(4)34-30(37)29(27(26)21-25-19-22(2)18-23(3)20-25)35-28(36)16-14-12-10-9-11-13-15-17-33-31(38)39-32(5,6)7/h18-20H,8-17,21H2,1-7H3,(H,33,38)(H,34,37)(H,35,36) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408893

(CHEMBL58914)Show SMILES CCc1c(Sc2cc(C)cc(C)c2)n(CCCCC(=O)NCCCCCCCCCCNC(=O)CCn2c(=O)c(C)cn(C3CC(N=[N+]=[N-])C(CO)O3)c2=O)c(=O)[nH]c1=O Show InChI InChI=1S/C42H61N9O8S/c1-5-32-38(55)46-41(57)50(40(32)60-31-23-28(2)22-29(3)24-31)20-15-12-16-35(53)44-18-13-10-8-6-7-9-11-14-19-45-36(54)17-21-49-39(56)30(4)26-51(42(49)58)37-25-33(47-48-43)34(27-52)59-37/h22-24,26,33-34,37,52H,5-21,25,27H2,1-4H3,(H,44,53)(H,45,54)(H,46,55,57) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408894
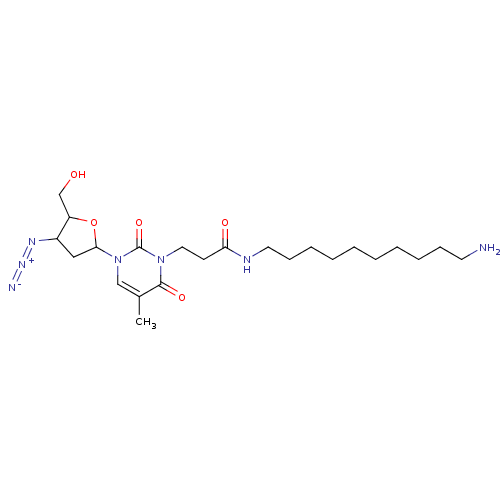
(CHEMBL292392)Show SMILES Cc1cn(C2CC(N=[N+]=[N-])C(CO)O2)c(=O)n(CCC(=O)NCCCCCCCCCCN)c1=O Show InChI InChI=1S/C23H39N7O5/c1-17-15-30(21-14-18(27-28-25)19(16-31)35-21)23(34)29(22(17)33)13-10-20(32)26-12-9-7-5-3-2-4-6-8-11-24/h15,18-19,21,31H,2-14,16,24H2,1H3,(H,26,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408895

(CHEMBL440482)Show SMILES CCc1c(Sc2cc(C)cc(C)c2)n(CCCCC(=O)NCCCCCCCCCCNC(=O)CCc2cn(C3CC(N=[N+]=[N-])C(CO)O3)c(=O)[nH]c2=O)c(=O)[nH]c1=O Show InChI InChI=1S/C41H59N9O8S/c1-4-31-38(55)46-40(56)49(39(31)59-30-22-27(2)21-28(3)23-30)20-14-11-15-34(52)43-18-12-9-7-5-6-8-10-13-19-44-35(53)17-16-29-25-50(41(57)45-37(29)54)36-24-32(47-48-42)33(26-51)58-36/h21-23,25,32-33,36,51H,4-20,24,26H2,1-3H3,(H,43,52)(H,44,53)(H,45,54,57)(H,46,55,56) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408896
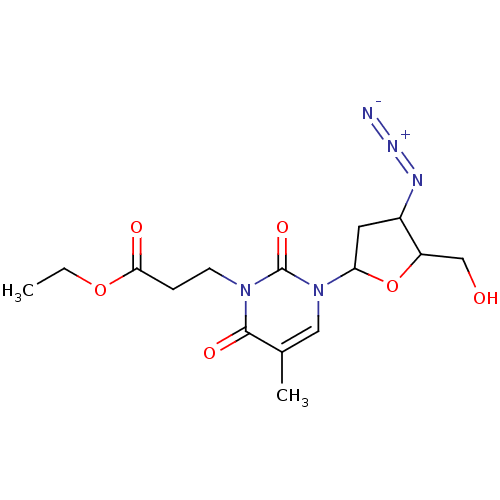
(CHEMBL56328)Show SMILES CCOC(=O)CCn1c(=O)c(C)cn(C2CC(N=[N+]=[N-])C(CO)O2)c1=O Show InChI InChI=1S/C15H21N5O6/c1-3-25-13(22)4-5-19-14(23)9(2)7-20(15(19)24)12-6-10(17-18-16)11(8-21)26-12/h7,10-12,21H,3-6,8H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408897

(CHEMBL262003)Show SMILES CCc1c(C)[nH]c(=O)c(NC(=O)CNC(=O)CNC(=O)CCCNC(=O)CCOCC2OC(CC2N=[N+]=[N-])n2cc(C)c(=O)[nH]c2=O)c1Cc1cc(C)cc(C)c1 Show InChI InChI=1S/C38H50N10O9/c1-6-26-24(5)43-37(54)35(27(26)15-25-13-21(2)12-22(3)14-25)44-33(52)18-42-32(51)17-41-30(49)8-7-10-40-31(50)9-11-56-20-29-28(46-47-39)16-34(57-29)48-19-23(4)36(53)45-38(48)55/h12-14,19,28-29,34H,6-11,15-18,20H2,1-5H3,(H,40,50)(H,41,49)(H,42,51)(H,43,54)(H,44,52)(H,45,53,55) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM10905

(3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...)Show InChI InChI=1S/C17H22N2O/c1-5-14-12(4)19-17(20)16(18)15(14)9-13-7-10(2)6-11(3)8-13/h6-8H,5,9,18H2,1-4H3,(H,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408899
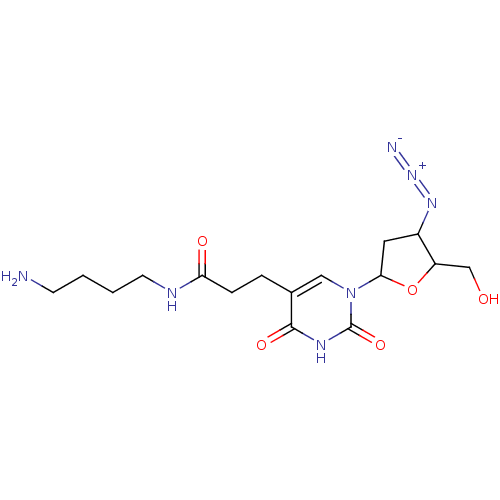
(CHEMBL300607)Show SMILES NCCCCNC(=O)CCc1cn(C2CC(N=[N+]=[N-])C(CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C16H25N7O5/c17-5-1-2-6-19-13(25)4-3-10-8-23(16(27)20-15(10)26)14-7-11(21-22-18)12(9-24)28-14/h8,11-12,14,24H,1-7,9,17H2,(H,19,25)(H,20,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408900
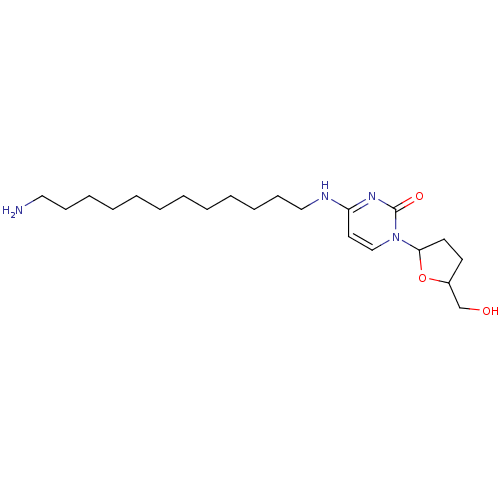
(CHEMBL417953)Show InChI InChI=1S/C21H38N4O3/c22-14-9-7-5-3-1-2-4-6-8-10-15-23-19-13-16-25(21(27)24-19)20-12-11-18(17-26)28-20/h13,16,18,20,26H,1-12,14-15,17,22H2,(H,23,24,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408901
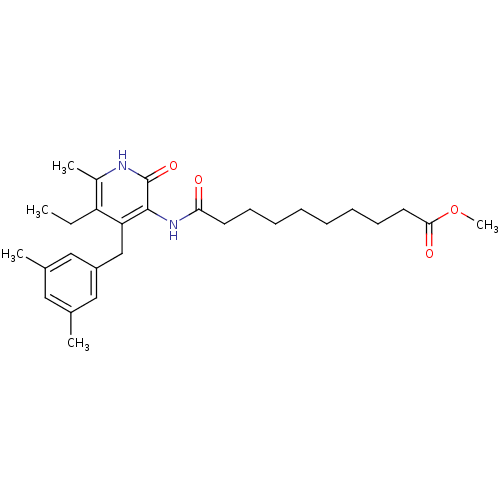
(CHEMBL60873)Show SMILES CCc1c(C)[nH]c(=O)c(NC(=O)CCCCCCCCC(=O)OC)c1Cc1cc(C)cc(C)c1 Show InChI InChI=1S/C28H40N2O4/c1-6-23-21(4)29-28(33)27(24(23)18-22-16-19(2)15-20(3)17-22)30-25(31)13-11-9-7-8-10-12-14-26(32)34-5/h15-17H,6-14,18H2,1-5H3,(H,29,33)(H,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408902

(CHEMBL58653)Show SMILES NCCCCCCCCCCNC(=O)CCc1cn(C2CC(N=[N+]=[N-])C(CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C22H37N7O5/c23-11-7-5-3-1-2-4-6-8-12-25-19(31)10-9-16-14-29(22(33)26-21(16)32)20-13-17(27-28-24)18(15-30)34-20/h14,17-18,20,30H,1-13,15,23H2,(H,25,31)(H,26,32,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50145605

(4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...)Show InChI InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408881

(CHEMBL418119)Show SMILES CCc1c(C)[nH]c(=O)c(NC(=O)CCCC(O)=O)c1Cc1cc(C)cc(C)c1 Show InChI InChI=1S/C22H28N2O4/c1-5-17-15(4)23-22(28)21(24-19(25)7-6-8-20(26)27)18(17)12-16-10-13(2)9-14(3)11-16/h9-11H,5-8,12H2,1-4H3,(H,23,28)(H,24,25)(H,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408880

(CHEMBL58213)Show SMILES CCc1c(Sc2cc(C)cc(C)c2)n(CCCCC(=O)OC)c(=O)[nH]c1=O Show InChI InChI=1S/C20H26N2O4S/c1-5-16-18(24)21-20(25)22(9-7-6-8-17(23)26-4)19(16)27-15-11-13(2)10-14(3)12-15/h10-12H,5-9H2,1-4H3,(H,21,24,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50408879
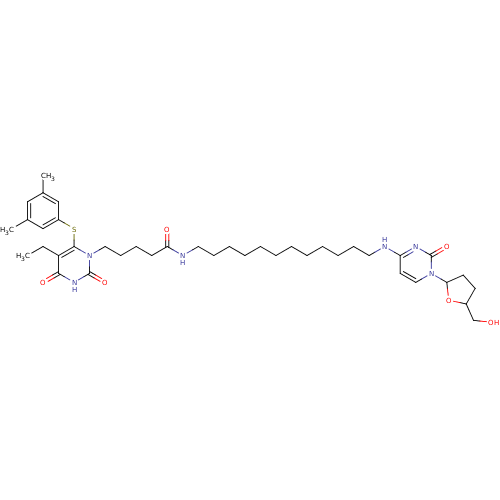
(CHEMBL20610)Show SMILES CCc1c(Sc2cc(C)cc(C)c2)n(CCCCC(=O)NCCCCCCCCCCCCNc2ccn(C3CCC(CO)O3)c(=O)n2)c(=O)[nH]c1=O Show InChI InChI=1S/C40H60N6O6S/c1-4-33-37(49)44-40(51)46(38(33)53-32-26-29(2)25-30(3)27-32)23-16-13-17-35(48)42-22-15-12-10-8-6-5-7-9-11-14-21-41-34-20-24-45(39(50)43-34)36-19-18-31(28-47)52-36/h20,24-27,31,36,47H,4-19,21-23,28H2,1-3H3,(H,42,48)(H,41,43,50)(H,44,49,51) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie
Curated by ChEMBL
| Assay Description
Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. |
J Med Chem 43: 1927-39 (2000)
BindingDB Entry DOI: 10.7270/Q2WS8VFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data